Ammonium tetrafluoroborate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 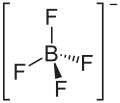
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium tetrafluoroborate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | NH 4 [BF 4 ] | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.84 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.85 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
220 ° C (sublimation) |
|||||||||||||||
| solubility |
soluble in water with slow decomposition |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium tetrafluoroborate is an inorganic chemical compound from the group of ammonium salts and fluoroborates . As a natural mineral formation , ammonium tetrafluoroborate is known under the name Barberiit .
Extraction and presentation
Ammonium tetrafluoroborate can be obtained by reacting boric acid with ammonium fluoride in sulfuric acid. The representation by reaction of ammonia with fluoroboric acid or ammonium bifluoride and boric acid is also possible.
properties
Ammonium tetrafluoroborate is a crystalline white odorless solid that is easily soluble in water. It slowly decomposes in water and its aqueous solution is acidic.
use
Ammonium tetrafluoroborate is used as the analytical reagent. It is also used in textile printing, the paint industry and as a catalyst. It is also used as a high-temperature flux in the metal industry, as a flame retardant and acts as a solid lubricant in cutting oil emulsions in aluminum rolling and forming.
Individual evidence
- ↑ a b c d e f g Entry on ammonium tetrafluoroborate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b Data sheet Ammonium tetrafluoroborate, 99.999% (metals basis) from AlfaAesar, accessed on July 9, 2016 ( PDF )(JavaScript required) .
- ↑ MA Malati: Experimental Inorganic / Physical Chemistry An Investigative, Integrated Approach to Practical Project Work . Elsevier, 1999, ISBN 978-1-78242-050-7 , pp. 64 ( limited preview in Google Book search).
- ^ Entry on ammonium tetrafluoroborate in the Hazardous Substances Data Bank , accessed on July 9, 2016.

![{\ displaystyle \ mathrm {NH_ {3} + HBF_ {4} \ longrightarrow NH_ {4} [BF_ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c7017709c8e9ceb53c236f5207b6caff56387c6f)