Baeyer-Villiger oxidation
The Baeyer-Villiger oxidation is a reaction in organic chemistry . Here, by reaction with percarboxylic a ketone to ester implemented:
Cyclic ketones are converted into lactones with ring expansion . The reaction is facilitated by the presence of Lewis acids , e.g. B. BF 3 , catalytically accelerated.
The Baeyer-Villiger oxidation is named after the German chemist Adolf von Baeyer (1835–1917) and the Swiss chemist Victor Villiger (1868–1934).
Reaction mechanism
A particularly suitable percarboxylic acid for the Baeyer-Villiger reaction is peroxytrifluoroacetic acid ( 2 ).
The ketone 1 and the percarboxylic acid 2 react to form the unstable tetrahedral intermediate 3 with a weak OO bond. After heterolytic cleavage of the OO bond, one of the alkyl groups (above: R) migrates to an O atom. An ester 4 is formed . In addition to the catalytic acceleration by Lewis acids , the character of the leaving group [above: trifluoroacetate ( 5 )] and the “migrating” group have a significant influence on the reaction rate.
In the case of unsymmetrically substituted ketones, the group that can better stabilize the positive charge of the carbenium ion migrates , if its migration is not sterically hindered.
The relative migration tendency from the highest to the lowest migration tendency:
tert - alkyl > sec- alkyl = phenyl > prim- alkyl> methyl
Experimental investigations of the Baeyer-Villiger oxidation of 18 O-labeled benzophenone showed that the carbonyl group of the ketone appears unchanged in the end product (phenyl benzoate):
Aldehydes
Aldehydes are usually oxidized to carboxylic acids, since in most cases an H atom shows the greatest tendency to migrate (for exceptions see Dakin reaction ). However, this reaction is of little importance in terms of preparation, since there are many other methods for the oxidation of aldehydes to carboxylic acids that are simpler and more atom-economical to implement.
Technical application
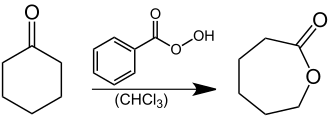
Caprolactone , a basic material for the production of polycaprolactone, a plastic from the group of thermoplastics , can be produced technically by a Baeyer-Villiger oxidation of cyclohexanone with peracids such as peracetic acid , perbenzoic acid or m -chloroperbenzoic acid. The oxidation can, however, also be brought about by means of catalysts using oxygen . In the laboratory, perbenzoic acid is used as an oxidizing agent:
Enzymatic variant
The use of Baeyer-Villinger monooxygenases (BVMO) allows the enantioselective synthesis of chiral lactones, the subclass of the cyclohexanone monooxygenases (CHMO) having proven to be particularly effective. So z. B. 4-methylcyclohexanone can be oxidized enantioselectively (> 96% ee ) to ( S ) -4-methyl-ε-caprolactone. The mechanism of the enzymatic Bayer-Villinger reaction was investigated in more detail.
Web links
Individual evidence
- ^ Siegfried Hauptmann : Organic chemistry . 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 366.
- ↑ a b Heinz GO Becker, Werner Berger, Günter Domschke, Egon Fanghänel , Jürgen Faust, Mechthild Fischer, Fritjof Gentz, Karl Gewald , Reiner Gluch, Roland Mayer , Klaus Müller, Dietrich Pavel, Hermann Schmidt, Karl Schollberg, Klaus Schwetlick, Erika Seiler, Günter Zeppenfeld: Organikum . 19th edition Johann Ambrosius Barth, 1993, ISBN 3-335-00343-8 .
- ↑ a b Paula Yurkanis Bruice: Organic Chemistry. 5th ed. Pearson Education, 2007, ISBN 978-3-8273-7190-4 , p. 831.
- ↑ Paula Yurkanis Bruice: Organic Chemistry. 5th Ed. Pearson Education, 2007, ISBN 978-3-8273-7190-4 , pp. 831-832.
- ↑ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry. Springer, 1972, ISBN 3-211-81060-9 , p. 258.
- ^ Paul S. Starcher, Benjamin Phillips: Synthesis of Lactones. In: Journal of the American Chemical Society. 80, No. 15, 1958, pp. 4079-4082, doi: 10.1021 / ja01548a063 .
- ^ SL Friess: Reactions of Per Acids. II. The Reaction of Perbenzoic Acid with Simple Cyclic Ketones. Kinetic Studies. In: Journal of the American Chemical Society . 71, No. 7, 1949, pp. 2571-2575, doi: 10.1021 / ja01175a093 .
- ↑ Sonia Horvat, Panagoitis Karallas, Jonathan M. White: Reactions of β-trimethylstannylcyclohexanones with peracids: investigations into the stannyl-directed Baeyer – Villiger reaction. In: Journal of the Chemical Society, Perkin Transactions 2. No. 10, 1998, pp. 2151-2154, doi: 10.1039 / a804427i .
- ↑ Carsten Bolm, Gunther Schlingloff, Konrad Weickhardt: Use of molecular oxygen in the Baeyer-Villiger oxidation the influence of metal catalysts. In: Tetrahedron Letters . 34, No. 21, 1993, pp. 3405-3408, doi : 10.1016 / S0040-4039 (00) 79167-2 .
- ↑ Shun-Ichi Murahashi, Yoshiaki Oda, Takeshi Naota: Fe 2 O 3 -catalyzed baeyer-villiger oxidation of ketones with molecular oxygen in the presence of aldehydes. In: Tetrahedron Letters. 33, No. 49, 1992, pp. 7557-7560, doi : 10.1016 / S0040-4039 (00) 60823-7 .
- ^ Iakov Polyak, Manfred T. Reetz , Walter Thiel: Quantum Mechanical / Molecular Mechanical Study on the Mechanism of the Enzymatic Baeyer-Villiger Reaction. In: Journal of the American Chemical Society. 134, No. 5, 2012, pp. 2732-2741, doi: 10.1021 / ja2103839 .