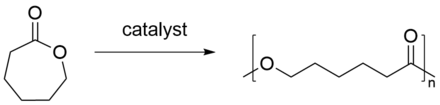Polycaprolactone
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| General | |||||||
| Surname | Polycaprolactone | ||||||
| other names |
|
||||||
| CAS number | 24980-41-4 | ||||||
| Monomer | ε-caprolactone | ||||||
| Molecular formula of the repeating unit | C 6 H 10 O 2 | ||||||
| Molar mass of the repeating unit | 114.14 g mol −1 | ||||||
| PubChem | 10401 | ||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.21 g cm −3 |
||||||
| Melting point |
58 ° C |
||||||
| Glass temperature |
−72 ° C |
||||||
| solubility |
up to 0.848 10 −2 mol% at 330 K and 22.4 MPa in supercritical CO 2 and ethanol (molecular weight: 1000 g mol −1 ) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polycaprolactone (PCL), more precisely poly-ε-caprolactone , is a biodegradable plastic that is made on the basis of petroleum . It is thermally deformable and is classified accordingly in the thermoplastics . It is formed as a chain of caprolactone , the ε- lactone of caproic acid .
construction
Polycaprolactone consists of a sequence of methylene units between which ester groups are formed. Due to this very simple structure, an almost unlimited rotation of the individual chain elements is possible, and the glass transition temperature T G , i.e. the curing temperature, is very low. At room temperature , short-chain, amorphous polycaprolactone is correspondingly soft and rubbery. Due to its uniform structure, however, it is easy to crystallize, which results in a reinforcement of the material.
synthesis
Polycaprolactone is produced by the ring-opening polymerization of ε- caprolactone under the action of heat and with the help of a catalyst , usually an alcohol or a diol :
A regular polymer is formed , about 50% of which crystallizes in the form of spherulites .
Material properties
In the crystalline polycaprolactone similar crystal structure to the polyethylene . It has a melting point of around 63 ° C, a tensile strength of 26 to 42 MPa and an elongation at break of 600 to 1000%. The glass transition temperature of the amorphous polymer is around −70 ° C, which is why it is rubbery at room temperature. However, polycaprolactone has a high tendency to crystallize, which occurs even when it is cooled down quickly, increasing the glass transition temperature to around −60 ° C. If the polymer has a high molar mass , i.e. it is a reaction product of many individual molecules, it becomes solid and flexible due to the partial crystallization; if the molecular mass is low, it becomes brittle.
Polycaprolactone is biodegradable. The degradation takes place by microorganisms , usually with the exclusion of oxygen ( anaerobic ). The material can be mixed well and also bonds with other plastics as well as with lignin , gels , starch and other materials. It also adheres to a wide variety of surfaces. It is non- toxic , easy to process, and easy to melt.
commitment
Polycaprolactone is used for a number of different applications because of its beneficial properties. In addition to classic areas of application for plastics such as packaging or the like, it is mainly used in the medical field. It is used for preparations with controlled release ( sustained release ) of drugs , adhesives and synthetic wound dressings and orthopedic impressions. In current research, it is also being researched as a carrier material for stem cells in regenerative osteogenesis or cartilage cells in tissue engineering .
Caprolactone polymers can be mixed with one another and are compatible with many other plastics, including polyethylene (PE), polypropylene (PP), polystyrene (PS), styrene-acrylonitrile copolymer (SAN), polycarbonate (PC) and polyethylene terephthalate (PET).
brand names
Polycaprolactone is manufactured in the USA by DOW and by the Swedish company Perstorp (former Solvay business).
Individual evidence
- ↑ F. Rezgui, M. Swistek, JM Hiver, C. G'Sell: Deformation and damage upon stretching of degradable polymers (PLA and PCL) . In: polymer . 46, No. 18, August, pp. 7370-7385. doi : 10.1016 / j.polymer.2005.03.116 .
- ↑ a b Entry on poly (ε-caprolactone) e. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ↑ Soraya Rodríguez Rojo, Ángel Martín, Elisa Sáez Calvo and María José Cocero: Solubility of Polycaprolactone in Supercritical Carbon Dioxide with Ethanol as Cosolvent . In: J. Chem. Eng. Data . 54, No. 3, 2009, pp. 962-965. doi : 10.1021 / je8007364 .
- ↑ a b c Datasheet Polycaprolactone, average M at Sigma-Aldrich , accessed on September 28, 2013 ( PDF ).
- ^ Marianne Labet, Wim Thielemans: Synthesis of polycaprolactone: a review . In: Chemical Society Reviews . 38, No. 12, 2009, pp. 3484-3504. doi : 10.1039 / B820162P . PMID 20449064 .
- ↑ Hansjörg Nitz: Thermoplastic compounds based on the renewable raw material lignin .
- ^ MD Lechner, K. Gehrke and EH Nordmeier: Makromolekulare Chemie , 4th edition, Birkhäuser Verlag, 2010, p. 129, ISBN 978-3-7643-8890-4 .
- ↑ B. Bogdanov: Low-temperature thermoplastics for use in orthopedics, in Orthopädie-Technik 2/03, 2003.
- ^ Joint German Congress of Orthopedics and Trauma Surgery: Production of an osteogenic polycaprolactone stem cell hydrogel construct for the reconstruction of segmental bone defects (2006).
- ↑ Tissue engineering of cartilage using a combination of long-term stable fibrin gel and polycaprolactone-based carrier material (PDF; 2.8 MB).