Benzatropine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
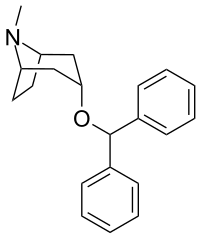
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Benzatropine | ||||||||||||
| other names |
(3- endo ) -3- (Diphenylmethoxy) -8-methyl-8-azabicyclo [3.2.1] octane |
||||||||||||
| Molecular formula | C 21 H 25 NO | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class |
Anticholinergic |
||||||||||||
| properties | |||||||||||||
| Molar mass | 307.43 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Benzatropine is a drug from the group of anticholinergics . It is used in the treatment of certain motor movement disorders , such as those that occur in Parkinson's disease or as a side effect of treatment with neuroleptics .
Benzatropine mesilate, the salt of benzatropine and methanesulfonic acid , is used pharmaceutically .
Mechanism of action
Benzatropine is a centrally acting anticholinergic. The combination of the tropine structure of atropine and the benzohydryl structure of diphenhydramine is responsible for the pharmacological effect. Benzatropine blocks the action of acetylcholine and regulates an imbalance between the neurotransmitters acetylcholine and dopamine , which enables its use in the treatment of the symptoms of early Parkinson's disease.
Animal experiments indicate that the anticholinergic effect is about half that of atropine, while the antihistamine effect corresponds to that of pyrilamine . Benzatropine also acts as a functional inhibitor of acid sphingomyelinase ( FIASMA ).
application areas
Benzatropine is indicated for the treatment of:
- drug parkinsonoid , akathisia, and acute dystonia
- Parkinson's Disease
- idiopathic or secondary dystonia
The study situation on the influence of anticholinergics on the occurrence of tardive dyskinesia as an undesirable late consequence of treatment with neuroleptics is inconsistent. Both favorable influence and no influence were found.
Side effects
Basically, side effects are observed with anticholinergics:
- Dry mouth
- blurred vision
- Memory problems
- Constipation
- Urinary retention
- Tachycardia
- anorexia
- Psychosis (overdose)
Trade names
- Cogentin
Individual evidence
- ↑ a b Datasheet Benztropine mesylate salt from Sigma-Aldrich , accessed on March 10, 2011 ( PDF ).
- ↑ External identifiers or database links for benzatropine mesilate: CAS number: 132-17-2, EC number: 205-048-8, ECHA InfoCard: 100.004.591 , ChemSpider : 10481911 , DrugBank : DB00245 , Wikidata : Q27105931 .
- ↑ MIMS Australia Pty Ltd. MIMS.
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of Novel Functional Inhibitors of Acid Sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .
- ↑ John M. Kane: Tardive Dyskinesia. In: Archives of General Psychiatry . 39, 1982, p. 473, doi : 10.1001 / archpsyc.1982.04290040069010 .
- ↑ Wszola BA, Newell KM, Sprague RL: Risk factors for tardive dyskinesia in a large population of youths and adults . In: Experimental and Clinical Psychopharmacology . 9, No. 3, 2001, pp. 285-296. doi : 10.1037 / 1064-1297.9.3.285 . PMID 11534539 .
- ^ Van Harten PN, Hoek HW, Matroos GE, Koeter M, Kahn RS: Intermittent neuroleptic treatment and risk for tardive dyskinesia: Curaçao Extrapyramidal Syndromes Study III . In: The American journal of psychiatry . 155, No. 4, 1998, pp. 565-567. PMID 9546009 .