Benzoyl bromide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
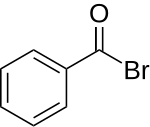
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Benzoyl bromide | ||||||||||||||||||
| other names |
Bromobenzoyl |
||||||||||||||||||
| Molecular formula | C 6 H 5 COBr | ||||||||||||||||||
| Brief description |
dark brown liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 185.02 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.57 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
−24 ° C |
||||||||||||||||||
| boiling point |
218-219 ° C |
||||||||||||||||||
| solubility |
reacts with water |
||||||||||||||||||
| Refractive index |
1.589 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Benzoyl bromide is a chemical compound from the group of benzoic acid derivatives .
Extraction and presentation
Benzoyl bromide can be obtained by reacting benzoyl chloride with bromotrimethylsilane .
properties
Benzoyl bromide is a dark brown liquid. The compound dissolves various metal bromides with electrolytic dissociation. Metal bromide hydrates can be dehydrated with benzoyl bromide.
use
Benzoyl bromide is a versatile reagent for the benzoylation of ethers . In the synthesis of carbohydrates as ethers (especially benzyl ethers ), protected hydroxyl groups can be converted into the corresponding benzoates under mild conditions in a one-pot reaction with benzoyl bromide and zinc triflate .
Individual evidence
- ↑ a b c d e f g h data sheet Benzoyl bromide, 97% from Sigma-Aldrich , accessed on March 25, 2019 ( PDF ).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2012, ISBN 978-1-4398-8049-4 , pp. 42 ( limited preview in Google Book search).
- ^ Richard Montgomery Stephenson: Handbook of the Thermodynamics of Organic Compounds . Springer Science & Business Media, 2012, ISBN 978-94-009-3173-2 , pp. 221 ( limited preview in Google Book search).
- ^ Alan R. Katritzky, Christopher J. Moody, Otto Meth-Cohn, Charles Wayne Rees: Comprehensive Organic Functional Group Transformations . Elsevier, 1995, ISBN 978-0-08-042326-5 , pp. 1334 ( limited preview in Google Book search).
- ↑ V. Gutmann, K. Utvary: reactions in anhydrous benzoyl. In: Monthly books for chemistry and related parts of other sciences. 90, 1959, p. 751, doi : 10.1007 / BF00902403 .
- ^ Tülay Polat, Robert J. Linhardt: Zinc triflate-benzoyl bromide: a versatile reagent for the conversion of ether into benzoate protecting groups and ether glycosides into glycosyl bromides . In: Carbohydrate Researche . tape 338 , no. 5 , 2003, p. 447-449 ( semanticscholar.org [PDF]).

