Lead (II) bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
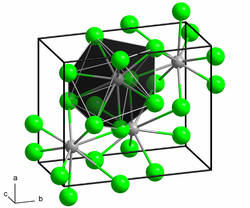
|
||||||||||||||||
| __ Pb 2+ __ Br - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Lead (II) bromide | |||||||||||||||
| other names |
Lead dibromide |
|||||||||||||||
| Ratio formula | PbBr 2 | |||||||||||||||
| Brief description |
odorless, white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 367.01 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.66 g cm −3 |
|||||||||||||||
| Melting point |
373 ° C |
|||||||||||||||
| boiling point |
916 ° C (other source 892 ° C) |
|||||||||||||||
| solubility |
in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lead (II) bromide is a lead salt which is used in the electronics industry and which is produced from 1,2-dibromoethane and tetraethyl lead when leaded gasoline is burned .
properties
Lead (II) bromide shows photoluminescence under UV light at low temperatures (<200 K). It crystallizes orthorhombically , space group Pmnb (space group no. 62, position 2) , with the lattice parameters a = 4.71 Å , b = 8.02 Å, c = 9.48 Å.
use
Lead (II) bromide is used in the electronics industry as a thin-film material. It can also be used for the electrolytic generation of lead (e.g. as a school experiment) and as a starting material for complex compounds .
See also
- Lead (IV) bromide (PbBr 4 , CAS number: 13701-91-2)
Individual evidence
- ↑ a b c d data sheet lead (II) bromide from AlfaAesar, accessed on January 7, 2010 ( PDF )(JavaScript required) .
- ↑ Data sheet lead (II) bromide from Sigma-Aldrich , accessed on March 13, 2011 ( PDF ).
- ^ CRC Handbook of Chemistry and Physics 67th edition 1986/87
- ↑ a b Entry on lead (II) bromide in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry lead compounds with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ WC de Gruijter, J. Kerssen: Luminescence of PbCl 2 and PbBr 2 single crystals II. Luminescence and EPR of uv irradiated crystals. In: Journal of Solid State Chemistry. 5, 1972, pp. 467-476, doi : 10.1016 / 0022-4596 (72) 90095-3 .
- ↑ W. Nieuwenkamp, JM Bijvoet: The crystal structure of lead bromide PbBr 2 . In: Zeitschrift für Kristallographie, Kristallgeometrie, Kristallphysik, Kristallchemie , 84, 132, pp. 49-61, doi : 10.1524 / zkri.1933.84.1.49 .


