Lead picrate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
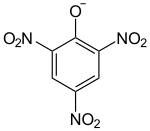
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lead picrate | ||||||||||||||||||
| other names |
2,4,6-trinitrophenol lead |
||||||||||||||||||
| Molecular formula | Pb (C 6 H 2 N 3 O 7 ) 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 663.41 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
explodes at approx. 270 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lead picrate is the common name of lead (II) -2,4,6-trinitrophenolate. It is the lead salt of picric acid and was used in the past and in some cases still today in detonators and primers .
properties
Lead picrate is in the form of yellow to orange-colored crystal needles. It is almost insoluble in water and somewhat soluble in acetone and ethanol . Lead picrate exists in many forms. There are forms that contain water of crystallization , but can be converted into the anhydrous form by heating. There are also various other forms such as basic lead picrate.
The connection is extremely sensitive to impact . A sample will explode if a 2 kg weight falls on it from a height of just 2 cm. This corresponds to an impact energy of 0.4 J. It is even more sensitive than mercury fulminate . It is therefore possible that a small amount will explode under the influence of its own weight. If lead picrate comes into contact with a flame, it evaporates with the development of fine lead dust. The compound is quite toxic, which is due on the one hand to the trinitrophenolate residue, but on the other hand also comes from the lead (II) ions .
presentation
Lead picrate is hardly produced today. However, the Germans produced it in large quantities during World War II . A readily water-soluble lead salt (e.g. lead nitrate ) was either mixed directly with picric acid , whereupon the poorly soluble lead picrate precipitated, or a solution of a readily water-soluble lead salt was mixed with a solution of a likewise readily water-soluble picrate, whereupon lead picrate also precipitated.
safety instructions
It should be noted that the formation of heavy metal picrates, especially lead picrate, should be avoided as a matter of principle. These often occur unintentionally when picric acid comes into contact with metals, their oxides , carbonates , hydroxides, etc. Numerous accidents in the past have shown that these connections are extremely dangerous and unstable. In Germany, possession, handling, storage, etc. is also subject to the Explosives Act (SprengG).
Individual evidence
- ↑ Makoto Matsukawa, Takehiro Matsunaga, Tadahiko Iwasaki, Masatake Yoshida, Shuzo Fujiwara: Synthesis and properties of lead picrates . In: Science and Technology of Energetic Materials . tape 65 , no. 1 , 2004, ISSN 1347-9466 , p. 7-13 .
- ↑ Entry on salts of picric acid in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Not explicitly in Regulation (EC) no. 1272/2008 (CLP) listed, but coincides with the specified marking under the group entries to lead compounds with the exception of those specified in this Annex and picric acid salts in the Classification and Labeling Inventory of the European Chemicals Agency ( ECHA), accessed on March 18, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ T. Urbanski: Chemistry and Technology of Explosives. Vol. 1, Pergamon Press - PWN Polish Scientific Publishers, 1964, p. 525.
literature
- Tadeusz Urbanski: Chemistry and Technology of Explosives. Volume III, Leipzig 1964.
- Rudolf Meyer: Explosives. 6th edition. VCH Verlagsgesellschaft, 1985, ISBN 3-527-26297-0 .



