Bromobenzyl cyanide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
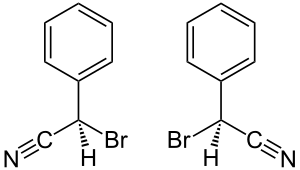
| ( R ) shape (left) and ( S ) shape (right) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Bromobenzyl cyanide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 6 BrN | |||||||||||||||
| Brief description |
yellowish solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 196.05 g mol −1 | |||||||||||||||
| Physical state |
solid, technically produced often also liquid |
|||||||||||||||
| density |
1.54 g cm −3 |
|||||||||||||||
| Melting point |
29 ° C |
|||||||||||||||
| boiling point |
242 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bromobenzyl cyanide is a chemical compound that is very irritating to the eyes . It was used as a chemical warfare agent.
Chemically, bromobenzyl cyanide is a stable compound; it is very resistant to hydrolysis . Pure bromobenzyl cyanide forms whitish to pale pink crystals , the technical product is a brown, oily liquid. It smells fruity. Because of its low volatility, bromobenzyl cyanide has a long duration of action. Its thermal instability and its detonation instability are disadvantageous. When heated, hydrogen cyanide can only develop in extreme cases . Bromobenzyl cyanide was used by the American side (CA, BBC) and the French side (Camite) at the end of the First World War . It is absorbed through the mucous membranes, eyes or breathing and leads to reddening of the skin and itching. Bromobenzyl cyanide is a strong eye irritant, even a concentration of 5 mg / m³ air is unbearable within a minute; the ICt 50 (concentration of the warfare agent which makes 50% of the exposed persons incapable of fighting) is 80–90 mg / m³ air in one minute of exposure. Immediate rinsing of the eye is imperative.
Bromobenzyl cyanide contains a stereocenter and is therefore chiral . The racemic bromobenzyl cyanide is a 1: 1 mixture of ( R ) -bromobenzyl cyanide and the mirror image of ( S ) -bromobenzyl cyanide.
See also
Individual evidence
- ↑ a b c d e f Entry on ALPHA-BROMOBENZYL CYANIDE in the Hazardous Substances Data Bank , accessed on January 1, 2008.
- ↑ Entry on bromobenzyl cyanide at TCI Europe, accessed on February 16, 2020.
- ↑ Bromobenzyl cyanide at Florochem. Retrieved March 14, 2020 .
- ^ National Academy of Sciences, National Research Council , Chemical-Biological Coordination Center, Review. Volume 5, p. 32, 1953.
- ↑ a b Entry on bromophenylacetonitrile in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 28, 2019.
- ^ National Defense Research Committee , Office of Scientific Research and Development, Progress Report. Vol. ND, Crc-132, August 1942.
- ^ Franke, S. et al .: Textbook of Military Chemistry , Volume 1, 2nd Edition, Military Publishing House of the GDR, Berlin (East), 1977.
