Bromine fluoride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
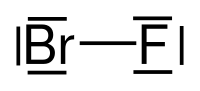
|
||||||||||
| General | ||||||||||
| Surname | Bromine fluoride | |||||||||
| other names |
|
|||||||||
| Molecular formula | BrF | |||||||||
| Brief description |
light red gas (≥ 20 ° C) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 98.9 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| density |
4.043 g l −1 |
|||||||||
| Melting point |
−33 ° C |
|||||||||
| boiling point |
20 ° C (decomposition) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Thermodynamic properties | ||||||||||
| ΔH f 0 |
−94 kJ / mol (gas) |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Bromine fluoride is an unstable interhalogen compound , selected from the elements bromine and fluorine exists. It is used as a bromination reagent.
Extraction and presentation
Bromine fluoride can be produced at 10 ° C by saturating bromine with fluorine.
Individual evidence
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 466.
- ↑ a b c d David R. Lide: CRC Handbook of Chemistry and Physics . 89th edition, Taylor & Francis, 2008, ISBN 978-1-420-06679-1 , pp. 4-53.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Erwin Riedel, Christoph Janiak: Inorganic Chemistry . 9th edition. Walter de Gruyter GmbH, Berlin 2015, ISBN 978-3-11-035526-0 , p. 431 .
- ^ JE Macintyre, FM Daniel, VM Stirling: Dictionary of inorganic compounds. CRC Press, 1992, ISBN 9780412301209 , p. 285.