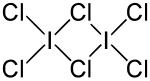
Iodine trichloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Iodine trichloride | |||||||||||||||
| Molecular formula |
|
|||||||||||||||
| Brief description |
red-brown crystals with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 233.26 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.12 g cm −3 |
|||||||||||||||
| Melting point |
33 ° C |
|||||||||||||||
| boiling point |
77 ° C (decomposition) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Iodine trichloride is a corrosive interhalogen compound consisting of iodine and chlorine . This was first portrayed by Humphry Davy in 1814 .
history
Emil von Behring experimented with numerous chemicals to find a cure for diphtheria . In some of his experimental animals, he was able to cure diphtheria with iodine trichloride - albeit with serious side effects . Von Behring then found the antitoxin against diphtheria toxin in the blood of these cured animals, which for the first time made it possible to treat true croup with an acceptable spectrum of side effects. With this he laid the foundation for passive immunization .
Extraction and presentation
Iodine trichloride is formed when chlorine is allowed to act on iodine chloride .
properties
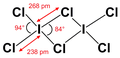
In contrast to other interhalogens, iodine trichloride forms a dimer , (ICl 3 ) 2 . The bond angles and bond lengths are not identical (see figure above). Iodine trichloride has a pungent smell and forms yellow needles that dissolve in the air. It dissolves well in alcohol , ether, and benzene .
Iodine trichloride reacts with water to form iodine chloride, hydrochloric acid and iodic acid .
use
Iodine trichloride can be used in preparative organic chemistry both for iodination and for chlorination , for example for aromatics.
Individual evidence
- ↑ a b c d e Data sheet iodine trichloride (PDF) from Merck , accessed on April 5, 2011.
- ↑ a b c d Bernd Dill, Fred Robert Heiker, Andreas Kirschning (eds.): Römpp Chemie Lexikon . 9th edition, Volume 3, Georg Thieme Verlag, 1992, ISBN 978-3-13-734809-2 , p. 2019.
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 459.
- ^ J. Simon: Emil Behring's Medical Culture: From Disinfection to Serotherapy . In: Med Hist. . 51, No. 2, 2007, pp. 201-218. PMC 1871706 (free full text).
- ↑ Behring, Emil: Investigations into the emergence of diphtheria immunity in animals , in: Deutsche Medicinische Wochenschrift 50, 1890, p. 11.