Cadmium selenate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
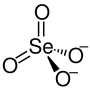
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cadmium selenate | |||||||||||||||
| Molecular formula | CdSeO 4 | |||||||||||||||
| Brief description |
colorless tablets (dihydrate) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 255.37 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.62 g cm −3 (dihydrate) |
|||||||||||||||
| Melting point |
100 ° C (decomposition, dihydrate) |
|||||||||||||||
| solubility |
soluble in water (70.5 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cadmium selenate is an inorganic chemical compound of cadmium from the selenate group .
Extraction and presentation
Cadmium selenate can be obtained by reacting cadmium oxide with concentrated selenic acid , whereby the monohydrate or dihydrate crystallizes out of the solution.
properties
As a dihydrate, cadmium selenate is a colorless solid in the form of transparent plates. When heated, this gives off water of crystallization from 100 ° C and converts to monohydrate. The monohydrate has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . The dihydrate has an orthorhombic crystal structure with the space group Pbca (space group no. 61) .
Individual evidence
- ↑ a b R. J. Meyer: Cadmium system number 33 . Springer-Verlag, 2013, ISBN 978-3-662-11295-3 , pp. 133 ( limited preview in Google Book search).
- ↑ a b c d William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 54 ( limited preview in Google Book search).
- ↑ Harmonized classification and labeling of selenium compounds with the exception of cadmium sulphoselenide and those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 18, 2017.
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entries on cadmium compounds, with the exception of cadmium sulphoselenide (xCdS.yCdSe), reaction mass of cadmium sulphide with zinc sulphide (xCdS.yZnS), reaction mass of cadmium sulphide with mercury sulphide (xCdS.yHgS), and those specified elsewhere in this Annex and selenium compounds with the exception of cadmium sulphoselenide and those specified in this Annex in the Classification and Labeling Inventory of elsewhere European Chemicals Agency (ECHA), accessed on March 18, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b C. Stålhandske: Structure of cadmium selenate monohydrate. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 37, p. 2055, doi : 10.1107 / S0567740881007942 .
- ↑ Huv bird: Chemist Calendar . Springer-Verlag, 2013, ISBN 978-3-662-06237-1 , p. 78 ( limited preview in Google Book search).



