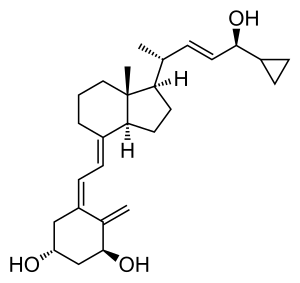
Calcipotriol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Calcipotriol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 27 H 40 O 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 412.61 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Calcipotriol is an artificially produced chemical compound from the group of secosteroids . It is used in medicine as a drug for topical treatment of psoriasis used (psoriasis). Calcipotriol is a synthetic derivative ( derivative ) of calcitriol .
Clinical information
Application areas (indications)
Externally, for the treatment of psoriasis (psoriasis): C. normalizes the cell growth and division activity of the skin cells .
Recent studies suggest that calcipotriol may also be effective for alopecia areata .
Contraindications (contraindications)
Diseases with changes in calcium metabolism, severe liver and kidney diseases.
Use during pregnancy and breastfeeding
Treatment during pregnancy and breastfeeding should be avoided as there is no experience.
Adverse effects (side effects)
Skin irritation (redness, itching, burning)
Mechanism of action (pharmacodynamics)
The effect is mediated via the vitamin D receptor. Vitamin D3 derivatives suppress the production of pro-inflammatory cytokines , which play a role in the psoriatic inflammatory process, and induce the formation of anti-inflammatory cytokines. In addition, vitamin D3 signal transduction interferes with other transcription factors that are considered to be important for the increased formation of inflammatory mediators in psoriasis vulgaris. A major part of the antipsoriatic effect is believed to be that keratinocyte proliferation is inhibited and the degree of differentiation is increased.
Other Information
Combination therapy
Calcipotriol and betamethasone
A fixed combination with corticosteroids is particularly useful at the beginning of the psoriasis treatment, as a faster onset of action can be achieved and at the same time possible irritative skin changes are suppressed. Therapy with the fixed combination of calcipotriol / betamethasone, which is only once a day, is very practical and highly tolerable. This fixed combination is recommended as initial therapy in the guidelines for medical treatment of scalp psoriasis.
Other combinations
Attention should be paid to the risk of a weakening of the effect with simultaneous use of salicylate-containing keratolytics or dithranol preparations as well as a possible increase in locally irritating effects when combined with vitamin A derivatives (tazarotene). Drug interactions: With simultaneous systemic use with calcium or vitamin D3, the serum calcium level should be checked regularly, as well as with drugs that can increase the calcium level in the serum. Therapy recommendation: For local therapy, especially maintenance therapy for mild to moderate psoriasis vulgaris, vitamin D3 derivatives are the first choice. In the first few weeks of treatment, a combination with topical corticosteroids as a fixed combination is superior to monotherapy in terms of effectiveness and tolerability.
History
In 1992, the first vitamin D 3 analog, calcipotriol, was approved for the topical treatment of mild to moderate psoriasis vulgaris. In 2002, the fixed combination preparation calcipotriol / betamethasone was approved for the initial treatment of psoriasis vulgaris.
literature
- S1 guideline : Psoriasis of the hairy head , registered guideline project, AWMF register number 013/074 ( online ), planned completion 12/2016
Trade names
- Monopreparations : Daivonex , Psorcutan
- Combination preparations : Daivobet ( gel or ointment ), Psorcutan Beta , Xamiol (gel), Enstilar (foam)
Web links
Individual evidence
- ↑ a b Datasheet Calcipotriol hydrate from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ↑ Entry on calcipotriol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ T. Narang, M. Daroach, MS Kumaran: Efficacy and safety of topical calcipotriol in management of alopecia areata: A pilot study. Dermatol Ther., January 30, 2017, doi : 10.1111 / dth.12464 PMID 28133875 .
- ↑ AA Čermáň, SS Solak, İ. Altunay, NA Küçükünal: Topical Calcipotriol Therapy for Mild-to-Moderate Alopecia Areata: A Retrospective Study. J Drugs Dermatol, June 14, 2015: 616-620. PMID 26091388 .
- ↑ A. Boonstra, FJ Barrat, C. Crain, VL Heath, HF Savelkoul, A. O'Garra: 1α, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4 + T cells to enhance the development of Th2 cells. In: Journal of Immunology (Baltimore, Md.: 1950). Volume 167, Number 9, November 2001, pp. 4974-4980, PMID 11673504 .
- ↑ A. Takeuchi, GS Reddy, T. Kobayashi, T. Okano, J. Park, S. Sharma: Nuclear factor of activated T cells (NFAT) as a molecular target for 1α, 25-dihydroxyvitamin D3-mediated effects. In: Journal of Immunology (Baltimore, Md.: 1950). Volume 160, Number 1, January 1998, pp. 209-218, PMID 9551973 .
- ↑ Kragballe K et al., Br J Dermatol 2006, 154: 1155-1160.
- ^ S1 guideline: Psoriasis of the hairy head, AWMF register number 013/074, as of 09/2009.