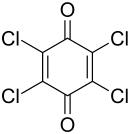
Tetrachloro- p -benzoquinone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrachloro- p -benzoquinone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 Cl 4 O 2 | |||||||||||||||
| Brief description |
yellow solid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 245.88 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
290–293 ° C (sublimation) |
|||||||||||||||
| solubility |
very sparingly soluble in water (0.25 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrachloro- p -benzoquinone is a chemical compound from the group of chlorinated benzoquinones .
Extraction and presentation
Tetrachloro- p -benzoquinone can be obtained by reacting p -benzoquinone with hydrogen chloride , whereby chlorohydroquinone and chloroquinone are formed as intermediate products .
It can also be made from trichlorophenol , often with PCDD contamination .
properties
Tetrachloro- p -benzoquinone is a flammable yellow solid with a characteristic odor, which is very sparingly soluble in water. At temperatures above 450 ° C, the compound decomposes, producing hydrogen chloride, carbon monoxide and carbon dioxide . It forms complexes with many non-aromatic electron donors, e.g. B. carbonyl compounds , esters, amides , lactones , lactams and alkyl iodides. It acts as a fungicide and is a powerful oxidizer.
use
Tetrachloro- p -benzoquinone is used as an oxidizing agent for dehydration .
Individual evidence
- ↑ a b c d e f g h Entry on tetrachloro-p-benzoquinone in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on Tetrachloro-p-benzoquinone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b Karl-Heinz Lautenschläger: Pocket Book of Chemistry - Karl-Heinz Lautenschläger . Harri Deutsch Verlag, 2007, ISBN 978-3-8171-1760-4 , pp. 295 ( limited preview in Google Book search).
- ↑ To Li, Shinsuke Tanabe, Guibin Jiang, John P. Giesy, Paul SK Lam: Persistent Organic Pollutants in Asia: Sources, Distributions, Transport and ... Elsevier, 2011, ISBN 0-08-055113-0 , p. 218 ( limited preview in Google Book search).
- ↑ Data sheet p-chloranil, 97% from AlfaAesar, accessed on July 22, 2013 ( PDF )(JavaScript required) .


