Chloride azide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
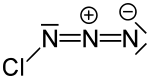
|
|||||||||||||
| General | |||||||||||||
| Surname | Chloride azide | ||||||||||||
| Molecular formula | ClN 3 | ||||||||||||
| Brief description |
colorless gas |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 77.47 g mol −1 | ||||||||||||
| Physical state |
gaseous |
||||||||||||
| Melting point |
−100 ° C |
||||||||||||
| boiling point |
−15 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Chlorine azide is a chemical compound from the group of azides or nitrogen halides .
Extraction and presentation
Chlorine azide can be obtained by reacting sodium azide with chlorine .
properties
Chlorine azide is a colorless, easily decomposable gas that smells of hypochlorous acid and is explosive when there are pressure fluctuations and when cooled (freezing out). It dissolves yellow-orange in carbon tetrachloride . Chlorine azide reacts slowly with hydrocarbons and other solvents . In its reactions it behaves like a compound with electropositive chlorine. It was first synthesized in 1908 by Friedrich Raschig . The CIN / NN / NN distances are 1.745 / 1.252 / 1.13 A, the CINN angle is 109 °.
Chlorine azide breaks down into nitrogen and chlorine at low pressure under a red glow when the gas is allowed to flow from a capillary into a heated tube. The spectrum of this glow shows a band system of four band groups.
Chlorine azide reacts with ammonia.
use
Chlorine azide is used in organic syntheses and as an initiator in chemical gas lasers.
Individual evidence
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 458.
- ↑ a b c Gurdeep Raj: Advanced Inorganic Chemistry Vol-1 . ISBN 81-87224-03-7 , pp. 745 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 685.
- ↑ W. Joe Frierson, AW Browne: . In: Journal of the American Chemical Society. 65, 1943, pp. 1698-1700, doi: 10.1021 / ja01249a013 .
- ↑ K. Gleu: The light emission during the decay of chloride azide . In: Journal of Physics . tape 38 , no. 3 , March 1926, p. 176-201 , doi : 10.1007 / BF01399108 .
- ↑ Stefan Bräse, Klaus Banert: Organic azide: Syntheses and Applications . John Wiley & Sons, 2010, ISBN 978-0-470-51998-1 , pp. 244 ( limited preview in Google Book search).
- ↑ Richard J. Lewis, Sr .: Hazardous Chemicals Desk Reference . John Wiley & Sons, 2008, ISBN 0-470-33445-2 , pp. 315 ( limited preview in Google Book search).



