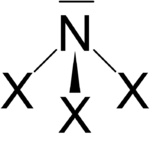Nitrogen halides
Nitrogen halides are binary compounds of nitrogen with halogens . Most of the known representatives have the composition NX 3 (with X = halogen), but halogen amines or halide hydrides and compounds with two or three nitrogen atoms per molecule are also known.
Nitrogen trihalides NX 3
The nitrogen trihalides, analogous to ammonia (NH 3 ), have a pyramidal structure with 3 equivalent halogen atoms. In addition to nitrogen trifluoride, these nitrogen halides are endothermic compounds that explode when heated or sometimes when touched. The following pyramidal nitrogen trihalides are known:
- Nitrogen trifluoride (NF 3 )
- Nitrogen trichloride (NCl 3 )
- Nitrogen tribromide (NBr 3 )
- Iodine nitrogen (nitrogen triiodide, NI 3 )
The stability, color and structure of the nitrogen trihalides also vary with the halogen involved:
- the colorless fluoride and the yellow chloride form relatively stable single molecules that only decompose when heated,
- the extremely unstable, red bromide can form both monomeric and polymeric modifications,
- the highly explosive iodide is only available as a black polymer .
Hydrogen Nitrogen Halides
In addition to binary halides, pyramidal hydrogen nitrogen halides or nitrogen halide hydrides of the types NHX 2 and NH 2 X, which are also referred to as halogen amines , have also been found . These are not binary compounds and in the strict sense not nitrogen halides, but most textbooks still include them in this group. This includes:
Monohalamines NH 2 X
- Monofluoramine (NH 2 F)
- Monochloramine (NH 2 Cl)
- Monobromamine (NH 2 Br)
- Monoiodamine (NH 2 I)
Dihalogenamine NHX 2
- Difluoroamine (NHF 2 )
- Dichloramine (NHCl 2 )
- Dibromoamine (NHBr 2 )
- Diiodamine (NHI 2 )
Higher nitrogen halides
In addition to the simple-pyramidal nitrogen trihalides and halogenamines derived from ammonia, there are also some compounds with two or three nitrogen atoms in the molecule. The dinitrogen tetrahalides N 2 X 4 have a bipyramidal structure, similar to ethane , but with one hydrogen atom replaced by a free electron pair or analogously to hydrazine . The dinitrogen dihalides N 2 X 2 have an N = N double bond and cis-trans isomers occur. The halogen azides N 3 X are derivatives of hydrazoic acid and have the same structure. The only stable, non-endothermic compound is dinitrogen tetrafluoride , all other compounds can only be represented at low temperatures and are sensitive to impact and temperature increases.
Dinitrogen tetrahalides N 2 X 4
- Dinitrogen Tetrafluoride (N 2 F 4 )
- Dinitrogen tetrachloride (N 2 Cl 4 )
Dinitrogen dihalides N 2 X 2
- Dinitrogen difluoride (N 2 F 2 )
- Dinitrogen dichloride (N 2 Cl 2 )
Halogen azides N 3 X
- Fluoroazide (N 3 F)
- Chlorine Azide (N 3 Cl)
- Bromine azide (N 3 Br)
- Iodine azide (N 3 I)
Overview of important properties
| Properties of the nitrogen halides | ||||
|---|---|---|---|---|
| Hydrogen Nitrogen Monohalides | ||||
| Surname | Monofluoroamine | Monochloramine | Monobromamine | Monoiodamine |
| Brief description | colorless gas | colorless gas | red-purple substance | black substance |
| Discovery (year, person) | 1988, Minkwitz | 1923, Marckwand, Wille | ? | 1962, Jander |
| Smp./Sdp. ( ° C ) | ~ −100 ° C (dec.) / - | ~ −70 ° C,> −110 ° C dec. | ? | > −90 ° C dec. |
| ΔH f 0 (kJ) (= standard enthalpy of formation ) | ? | 390 kJ | ? | ? |
| Hydrogen Nitrogen Dihalides | ||||
| Surname | Difluoroamine | Dichloramine | Dibromoamine | Diiodamine |
| Brief description | colorless gas | yellow gas | orange substance | black substance |
| Discovery (year, person) | 1931, Ruff | 1929, Chapin | 1958, Jander | 1962, Jander |
| Smp./Sdp. ( ° C ) | −116.4 ° C / −23.6 ° C | decomposition | ? | > −60 ° C dec. |
| ΔH f 0 (kJ) | −67 kJ | ? | ? | ? |
| Nitrogen trihalides | ||||
| Surname | Nitrogen trifluoride | Nitrogen trichloride | Nitrogen tribromide | Nitrogen triiodide |
| Brief description | colorless gas | yellow oil | deep red crystals | deep red substance |
| Discovery (year, person) | 1928, Ruff | 1811, Dulong | 1975, Jander | 1990, Klapötke |
| Smp./Sdp. ( ° C ) | −206.8 ° C / −129.0 ° C | −40 ° C / 71 ° C | > −100 ° C explosion | > −78 ° C dec. |
| ΔH f 0 (kJ) | −125 kJ | + 229 kJ | ? | + 290 kJ |
| Dinitrogen tetrahalides | ||||
| Surname | Nitrous tetrafluoride | Nitrous tetrachloride | Dinitrogen tetrabromide | Dinitrogen tetraiodide |
| Brief description | colorless gas | ? | - | - |
| Discovery (year, person) | 1957, Colburn | ? | - | - |
| Smp./Sdp. ( ° C ) | −164.5 ° C / −73 ° C | ? | - | - |
| ΔH f 0 (kJ) | ? | ? | - | - |
| Dinitrogen Dihalides | ||||
| Surname | Dinitrogen difluoride | Nitrous dichloride | Nitrous dibromide | Nitrous diiodide |
| Brief description | colorless gas | ? | - | - |
| Discovery (year, person) | 1942, Haller | ? | - | - |
| Smp./Sdp. ( ° C ) | trans: −172 ° C / −114.4 ° C cis: −195 ° C / −105.7 ° C |
? | - | - |
| ΔH f 0 (kJ) | trans: 82.1 kJ cis: 69.5 kJ |
? | - | - |
| Tri-nitrogen monohalides / halogen azides | ||||
| Surname | Fluoroazide | Chloride azide | Bromine azide | Iodine azide |
| Brief description | green-yellow gas | colorless gas | orange-red liquid | colorless substance |
| Discovery (year, person) | 1942, Haller | 1908, Raschig | 1925, Spencer | 1900, Hantzsch |
| Smp./Sdp. ( ° C ) | −154 ° C / −82 ° C | −100 ° C / −15 ° C | −45 ° C / explosion | ~ 20 ° C / explosion |
| ΔH f 0 (kJ) | ? | + 390 kJ | + 385 kJ | ? |
| "?" = Value not known , "-" = substance not known | ||||
Individual evidence
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 678-688.
- ↑ a b c Nikolaus Korber: nitrogen halides , inorganic chemistry, lecture script, University of Regensburg
- ↑ HP Latscha, HA Klein: Inorganic Chemistry . Springer, 2002, ISBN 3-540-42938-7 , p. 312ff.
- ^ J. Jander: Non-Aqueous Solvents for Preparation and Reactions of Nitrogen Halogen Compounds . (PDF; 465 kB) Pure Appl. Chem. , Vol. 49, Pergamon, 1977, pp. 67-73.
- ↑ a b c d e connection has not yet been detected.