Nitrogen tribromide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
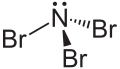
|
|||||||||||||
| General | |||||||||||||
| Surname | Nitrogen tribromide | ||||||||||||
| other names |
Tribromoamine |
||||||||||||
| Molecular formula | NBr 3 | ||||||||||||
| Brief description |
dark red, explosive solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 253.72 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
explodes> −100 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Nitrogen tribromide is a chemical compound that belongs to the group of nitrogen halides . Due to its sensitivity and high explosiveness, there is no practical application for the connection.
Occurrence
If bromine is used instead of chlorine to disinfect swimming pools , the reaction with urea can produce nitrogen tribromide as a by-product from human excretions.
Extraction and presentation
Nitrogen tribromide can be obtained by bromination of nitrogen trichloride
or by reacting ammonium bromide , sodium chlorite and iron (III) bromide
or by reaction of bis (trimethylsilyl) bromoamine with bromine chloride at -85 ° C in n-pentane as solvent
Nitrogen tribromide can also be produced by electrolysis of an ammonium iodide and potassium bromide solution (NH 4 I + KBr).
In the explosion, nitrogen tribromide breaks down into the elements:
literature
- Jochen Jander: Non-Aqueous Solvents for Preparation and Reactions of Nitrogen Halogen Compounds (PDF; 477 kB) . Pure & Appl. Chem., Vol. 49, pp. 67-73.
Individual evidence
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 702.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Lenntech: Bromine as Disinfectant
- ^ Water Quality and Health Council: Pool Health
- ↑ Lateral Science: Fulminating oils ( Memento of the original of September 21, 2008 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Jochen Jander: Non-Aqueous Solvents for Preparation and Reactions of Nitrogen Halogen Compounds (PDF; 477 kB) . Pure Appl. Chem. , Vol. 49, pp. 67-73.



![{\ displaystyle \ mathrm {[(CH_ {3}) _ {3} Si] _ {2} NBr + 2 \ BrCl \ longrightarrow NBr_ {3} +2 \ (CH_ {3}) _ {3} SiCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f4d42e052dade985c4cdac69b9fdcc6310e346ac)
