Fluoroazide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
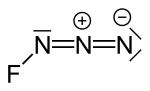
|
|||||||||||||
| General | |||||||||||||
| Surname | Fluoroazide | ||||||||||||
| other names |
Triazadienyl fluoride |
||||||||||||
| Molecular formula | N 3 F | ||||||||||||
| Brief description |
green-yellow gas |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 61.02 g mol −1 | ||||||||||||
| Physical state |
gaseous |
||||||||||||
| density |
1.3 g cm −3 |
||||||||||||
| Melting point |
−132.5 ° C |
||||||||||||
| boiling point |
−82 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Fluorazide is a chemical compound of fluorine from the group of halogen azides or nitrogen halides .
history
Among the halogen azides, fluoroazide occupies a special position because its N 3 group is positively polarized. As the last compound in this class of substances, it was synthesized in 1942 by John F. Haller by reacting HN 3 with fluorine . Since fluorazide decomposes in the gas phase at room temperature and explodes extremely easily in the condensed state, it was not until the 1990s that it was presented in its pure form and more closely characterized, as well as the precise determination of spectroscopic data and values for melting and boiling points.
Extraction and presentation
Azide can be prepared by reaction of hydrazoic acid with fluorine are recovered.
It can also be made by reacting sodium azide with fluorine.
properties
Fluoroazide is a greenish yellow gas that is unstable at 25 ° C and breaks down into nitrogen (I) fluoride and nitrogen .
In the liquid and solid forms, the compound is extremely explosive. At room temperature, it did not react with water , oxygen , xenon difluoride , oxygen difluoride and trimethylsilyl azide . Reactions with nitrogen monoxide , carbon monoxide , carbonyl sulfide lead to products that can be interpreted as intermediate products via the formation of the NF {a 1 Δ} radical.
At temperatures between 40 and 80 ° C it breaks down into nitrogen (I) fluoride and nitrogen (III) fluoride . It reacts with chlorine between 50 and 85 ° C to form chlorodifluoramine and nitrogen.
The UV / Vis absorption spectrum of gaseous fluorazide shows three absorptions λ max = 414, 208 and <190 nm, ε max = 12,800 and> 1200L mol −1 cm −1 , analogous to that of other halogen azides . The mass spectrum shows an ionization energy of 70 eV and an ion source temperature of 50 ° C. It was assumed that due to the low NF binding energy (<150 kJ / mol) in fluorazide, a fluorine ion could be split off with the formation of an N 3+ salt. This would have made it possible to analyze the structural, spectroscopic and physico-chemical properties of this non-metal cation, which was previously inaccessible in preparative terms. However, it was found that the fluoride donor properties are not sufficient to form the corresponding N 3+ salts in the presence of the Lewis acids boron trifluoride or arsenic pentafluoride . Rather, only those adducts are formed that have a coordinative (N α → M) bond (M = B, As). The atomic distances in the molecule are FN 144.4 pm, FN-NN 125.3 pm and FNN-N 113.2 pm, the angles FNN 103.8 ° and NNN 170.9 °. Two cyclic isomers of the compound are also known in which the three nitrogen atoms form a ring.
use
Fluorazide dissociates exothermically into nitrogen and electronically excited NF molecules, so that it can possibly be used to operate high-energy chemical lasers .
Individual evidence
- ↑ a b c d e Naumann: Fluorine and fluorine compounds . Springer-Verlag, 2013, ISBN 978-3-642-72344-5 , p. 62 ( limited preview in Google Book search).
- ↑ Brener, Nathan E .; Kestner, Neil R .; Callaway, Joseph (December 1990). Theoretical Studies of Highly Energetic CBES Materials: Final Report for the Period 2 March 1987 to 31 May 1987 (PDF). Louisiana State University, Department of Physics and Astronomy. pp. 21-27. Retrieved June 25, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Gabriele Schatte, Helge Willner: The interaction of N3F with Lewis acids and HF. N3F as a possible precursor for the synthesis of N 3+ salts / The Interaction of N3F with Lewis-Acids and HF. N3F as Possible Precursor for the Synthesis of N3 + Salts. In: Zeitschrift für Naturforschung B. 46, 1991, doi : 10.1515 / znb-1991-0410 .
- ↑ a b c Khodayar Gholivand, Gabriele Schatte, Helge Willner: Properties of triazadienyl fluoride, N3F. In: Inorganic Chemistry. 26, 1987, p. 2137, doi : 10.1021 / ic00260a025 .
- ↑ DJ Benard, BK Winker, TA Seder, RH Cohn: Production of nitrogen monofluoride (a1.DELTA.) By dissociation of fluorine azide. In: The Journal of Physical Chemistry. 93, 1989, p. 4790, doi : 10.1021 / j100349a022 .
- ↑ Peter Merlet: Gmelin Handbook of Inorganic Chemistry - Fluorine Compounds with Oxygen and Nitrogen . Springer Science & Business Media, 2013, ISBN 978-3-662-06339-2 , pp. 406 ( limited preview in Google Book search).
- ↑ Dines. Christen, HG Mack, G. Schatte, H. Willner: Structure of triazadienyl fluoride, FN3, by microwave, infrared, and ab initio methods. In: Journal of the American Chemical Society. 110, 1988, p. 707, doi : 10.1021 / ja00211a007 .
- ↑ Galina Chaban, David R. Yarkony, Mark S. Gordon: On the structure and stability of geometrical isomers of N3F. In: The Journal of Chemical Physics. 103, 1995, p. 7983, doi : 10.1063 / 1.470216 .


![{\ displaystyle \ mathrm {N_ {3} F \ longrightarrow [NF] + N_ {2} \ longrightarrow N_ {2} F_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/04d2bd13b186814b4cef45fc00d777f6ee40c6f1)

![{\ displaystyle \ mathrm {N_ {3} F + CO \ longrightarrow [FNCO] + N_ {2} \ longrightarrow FC (O) NCO}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6f362ce76cd40ca8772f75c4ab83183797be8d65)

