Nitrogen trifluoride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nitrogen trifluoride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | NF 3 | |||||||||||||||
| Brief description |
colorless gas with a musty odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 71.00 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
1.89 g cm −3 (−129 ° C) |
|||||||||||||||
| Melting point |
−208.5 ° C |
|||||||||||||||
| boiling point |
−129 ° C |
|||||||||||||||
| solubility |
very bad in water (61 mg l −1 at 20 ° C) |
|||||||||||||||
| Dipole moment | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 10 ml m −3 or 30 mg m −3 |
|||||||||||||||
| Global warming potential |
19700 (based on 100 years) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nitrogen trifluoride is a chemical compound that belongs to the group of nitrogen halides .
history
Nitrogen trifluoride was first produced in 1928 by Otto Ruff (1871–1939) by electrolysis of anhydrous ammonium hydrogen difluoride (NH 4 HF 2 ). In 1957, succeeded Peter Sartori , to make the process much safer by the resulting by-products unstable and explosive substances fluoramine NH 2 F and difluoramine NHF 2 by introduced during the reaction Braunstein eliminated.
Extraction and presentation
It can be produced by the catalytic conversion of ammonia with fluorine or by electrolysis of molten ammonium hydrogen fluoride:
The representation from the elements, however, is only possible under unusual conditions (i.e. electrical discharges at high pressure and high temperatures).
properties
Physical Properties
Nitrogen trifluoride has a trigonal - pyramidal structure, with the nitrogen atom located at the top of the pyramid. The bond lengths and angles are shown in the picture.
Chemical properties
Nitrogen trifluoride does not react with water at room temperature and, in contrast to ammonia, has almost no basic properties. It is a powerful oxidizer . It reacts with aluminum chloride to form aluminum fluoride :
Environmental characteristics and occurrence
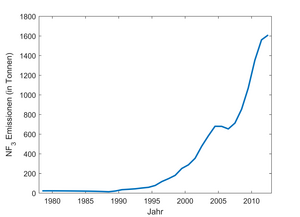
Nitrogen trifluoride as a greenhouse gas has a global warming potential of 19,700 over a time horizon of 100 years . One kilogram of nitrogen trifluoride warms the climate 19,700 times as much as one kilogram of carbon dioxide . Nitrogen trifluoride has an atmospheric lifetime of 509 ± 21 years. In the atmosphere it is mainly broken down by photolysis (approx. 70%) and by reaction with oxygen radicals (approx. 30%). In 2008, the nitrogen trifluoride concentration in the troposphere was 0.45 ppt (parts per trillion air parts), in 2011 it was 0.86 ppt and in 2016 it was just under 1 ppt. The concentration is significantly higher in the northern hemisphere than in the southern hemisphere. This result fits the localization of the main emitters in the northern industrial countries. Nitrogen trifluoride emissions have risen sharply since the late 1970s. While the annual emissions in the 1980s were around 20 tons, in 2000 it was already around 290 tons and in 2012 around 1610 tons. The nitrogen trifluoride emissions from 2012 correspond to around 32 million tons of carbon dioxide in terms of their greenhouse effect.
In view of the high greenhouse gas potential, scientists have recommended adding nitrogen trifluoride to the list of dangerous greenhouse gases, the emissions of which are monitored under the Kyoto Protocol . At the UN climate conference in Doha in 2012 , the chemical was finally added to the list of greenhouse gases on December 8, 2012 in an addendum to the Kyoto Protocol. This supplement applies from the beginning of the second commitment period - Kyoto II - until 2020.
In Germany, the release of nitrogen trifluoride has been statistically recorded since 2015. In 2016, 38 tons were sold to users in Germany, which is almost 58% less than in the previous year.
use
Nitrogen trifluoride is used in the semiconductor and in very large quantities in the liquid crystal display - and solar industry for cleaning of the PECVD -Beschichtungskammern of silicon dioxide , silicon oxynitride and silicon nitride - residues used. The sharp rise in nitrogen trifluoride concentration in the atmosphere is due to these applications. The replacement by toxic but environmentally friendly fluorine is being tested on an industrial scale. Furthermore, there was a time in military high-energy hydrogen fluoride - lasers (. Eg MIRACL ) for use, was used as the oxidizer used in rocket fuel, as an additive to incandescent lamps tested -Gasfüllungen and for the production of extremely toxic tetrafluorohydrazine (N 2 F 4 ) - a Another rocket fuel component and undesirable by-product of nitrogen trifluoride production - used.
production
In total, around 4,000 tons of nitrogen trifluoride were produced in 2008. The world's largest manufacturer by far is the American chemical company Air Products & Chemicals Inc. If the entire annual nitrogen trifluoride production were released into the earth's atmosphere, the resulting greenhouse effect would be the same as that produced by the release of around 79 million tons of CO 2 . In fact, in 2008 around 16% of annual production ended up in the atmosphere. By 2011 this proportion had fallen to around 10%.
safety instructions
According to EC directives, nitrogen trifluoride is classified as oxidizing and harmful . It decomposes when heated and reacts violently with some organic compounds (e.g. flammable substances).
literature
- O. Ruff, F. Luft, J. Fischer: Z. anorg. allg. Chem. 172, 1928, p. 417 ff.
- O. Ruff, Z. anorg. allg. Chem. 197, 1931, p. 273 ff.
- O. Ruff, L. Staub: Z. anorg. general Chem. 198, 1932, p. 32 ff.
- G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. 2nd Edition. Volume 1, Academic Press 1963, ISBN 0-12-126601-X , pp. 181-183.
Individual evidence
- ↑ a b c d e f g Entry on nitrogen trifluoride in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-51.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 7783-54-2 or nitrogen trifluoride ), accessed on November 2, 2015.
- ↑ a b c d A. Totterdill, T. Kovács, W. Feng, S. Dhomse, CJ Smith: Atmospheric lifetimes, infrared absorption spectra, radiative forcings and global warming potentials of NF3 and CF3CF2Cl (CFC-115) . In: Atmos. Chem. Phys. tape 16 , no. 17 , September 14, 2016, ISSN 1680-7324 , p. 11451-11463 , doi : 10.5194 / acp-16-11451-2016 .
- ↑ Patent for Electrolytic anode and method for electrolytically synthesizing fluorine containing substance using the electrolytic anode
- ↑ Lecture for the group of German fluorochemicals (PDF; 344 kB)
- ↑ HP Latscha, HA Klein: Inorganische Chemie , 2002, Springer, ISBN 3-540-42938-7 , p. 312 ff.
- ↑ Oskar Glemser, Johann Schröder, Joachim Knaak: Note on the representation of nitrogen trifluoride by electrolysis of molten ammonium hydrogen fluoride. In: Chemical Reports . Volume 99, No. 1, pp. 371-374, January 1966. doi: 10.1002 / cber.19660990157 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 695.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 697.
- ^ A b M. Rigby, RG Prinn, S. O'Doherty, BR Miller, D. Ivy: Recent and future trends in synthetic greenhouse gas radiative forcing . In: Geophysical Research Letters . tape 41 , no. 7 , April 16, 2014, ISSN 1944-8007 , p. 2013GL059099 , doi : 10.1002 / 2013GL059099 .
- ↑ a b c d Tim Arnold, Christina M. Harth, Jens Mühle, Alistair J. Manning, Peter K. Salameh: Nitrogen trifluoride global emissions estimated from updated atmospheric measurements . In: Proceedings of the National Academy of Sciences . tape 110 , no. 6 , February 5, 2013, ISSN 0027-8424 , p. 2029-2034 , doi : 10.1073 / pnas.1212346110 , PMID 23341630 , PMC 3568375 (free full text).
- ↑ a b c Ray F. Weiss, Jens Mühle, Peter K. Salameh, Christina M. Harth: Nitrogen trifluoride in the global atmosphere . In: Geophysical Research Letters . tape 35 , no. 20 , October 1, 2008, ISSN 1944-8007 , p. L20821 , doi : 10.1029 / 2008GL035913 .
- ^ Doha Amendment to the Kyoto Protocol - home page . United Nations, Framework Convention on Climate Change.
- ↑ Doha Amendment to the Kyoto Protocol (PDF, English; 120 kB) . United Nations, Framework Convention on Climate Change. Retrieved March 27, 2013.
- ↑ Destatis: Sales of sulfur hexafluoride increased in 2016 . In UmweltMagazin . 47, No. 6, 2017, ISSN 0173-363X , p. 12.
- ↑ Underestimated greenhouse gas. Wissenschaft.de, July 3, 2008, accessed September 8, 2019 .
- ↑ J. Oshinowo, A. Riva, M Pittroff, T. Schwarze and R. Wieland: Etch performance of Ar / N 2 / F 2 for CVD / ALD chamber clean . In: Solid State Technology . 52, No. 2, 2009, pp. 20-24.