Monofluoroamine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
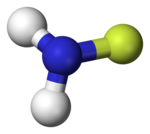
|
|||||||||||||
| General | |||||||||||||
| Surname | Monofluoroamine | ||||||||||||
| other names |
Fluoramine |
||||||||||||
| Molecular formula | NH 2 F | ||||||||||||
| Brief description |
unstable gas |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 35.02 g mol −1 | ||||||||||||
| Physical state |
gaseous |
||||||||||||
| density |
1.431 g l −1 |
||||||||||||
| Melting point |
−100 ° C |
||||||||||||
| boiling point |
−77 ° C |
||||||||||||
| solubility |
soluble in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Monofluoramine , NH 2 F, is a colorless, decomposable gas with a melting point of around −100 ° C. It was first synthesized in 1988 by Rolf Minkwitz .
The monofluoramine has a pyramidal structure and C s symmetry. It already decomposes at around −100 ° C:
The decomposition takes place in the same way as dichloramine .
Analogous to monochloramine , monofluoramine is a strong aminating agent:
Monofluoramine is a weak base and forms HX salts of the form NH 3 F + X - with strong acids , whereby the NH 3 F + is referred to as the fluorammonium ion.
The synthesis takes place in a high vacuum from the hydrofluoric acid salt NH 3 F + HF 2 - . This is evaporated and passed over potassium fluoride powder. The HF is absorbed. The monofluoroamine can be condensed and isolated as a colorless solid in liquid nitrogen .
literature
- AF Holleman , N. Wiberg : Inorganic Chemistry . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 790 (reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
Individual evidence
- ^ A b c William M. Haynes: CRC Handbook of Chemistry and Physics . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 63 ( limited preview in Google Book search).
- ↑ a b Gurdeep Ra: Advanced Inorganic Chemistry: Volume II . 12th edition. Krishna Prakashan Media, 2010, ISBN 978-81-8283-776-8 , pp. 162 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Rolf Minkwitz, Rüdiger Naß: Presentation and Properties of Monofluoramine, H 2 NF / Preparation and Properties of Monofluoramine, H 2 NF. In: Zeitschrift für Naturforschung B. 43, 1988, p. 1478, doi: 10.1515 / znb-1988-1114 .
- ^ A b Holleman / Wiberg: Basics and main group elements . Walter de Gruyter GmbH & Co KG, 2016, ISBN 978-3-11-049585-0 , p. 790 ( limited preview in Google Book search).

