Monochloramine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
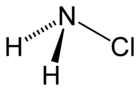
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Monochloramine | |||||||||||||||
| other names |
Chloramine |
|||||||||||||||
| Molecular formula | NH 2 Cl | |||||||||||||||
| Brief description |
colorless to yellowish liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 51.48 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| Melting point |
approx. −70 ° C (97%, contains NH 3 ) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Monochloramine is a chemical compound that is used as a disinfectant and belongs to the group of chloramines or nitrogen halides . In its pure form, monochloramine is unstable and decomposes at temperatures above −110 ° C; from −40 ° C the decomposition proceeds violently to explosively. It is stable at low pressures and in dilute solutions.
Occurrence
Monochloramine can be obtained by reacting chlorine with nitrogen-containing compounds e.g. B. arise in swimming pools. Along with other chloramines, it is responsible for the typical swimming pool odor and can irritate the eyes and mucous membranes.
Extraction and presentation
Monochloramine can be obtained by reacting ammonia (or ammonia compounds) with hypochlorous acid under slightly alkaline conditions:
The use of chlorine is less advisable due to the formation of explosive nitrogen trichloride as a by-product.
use
Monochloramine is used in low concentrations in the USA as a disinfectant in water supply systems as an alternative to chlorination . Furthermore, monochloramine forms an important intermediate product in the hydrazine synthesis according to the Raschig process . Monochloramine forms blue indophenol dyes with phenols in the presence of a catalyst , which can be used as evidence.
safety instructions
Monochloramine is toxic to certain fish species.
Individual evidence
- ↑ a b Entry on chloramide in the GESTIS substance database of the IFA , accessed on February 28, 2017(JavaScript required) .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of chloramides in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on September 27, 2017, is reproduced from a self-classification by the distributor .
- ↑ Hygiene requirements for bathrooms and their monitoring . In: Federal Health Gazette . tape 57 , no. 2 , 2014, p. 258–279 , doi : 10.1007 / s00103-013-1899-7 (free full text).
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance . Springer Fachmedien, Wiesbaden 2015, ISBN 978-3-658-07310-7 , p. 188 , doi : 10.1007 / 978-3-658-07310-7 .
- ↑ Gordon M. Fair, J. Carrell Morris, Shih Lu Chang, Ira Weil, Robert P. Burden: Behavior of chlorine as a water disinfectant . In: Journal of the American Water Works Association . tape 40 , no. 10 , 1948, pp. 1051-1061 , JSTOR : 41234959 .
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 477-479.
- ^ Gregory L. Seegert, Arthur S. Brooks, John R. Vande Castle, Kenneth Gradall: The Effects of Monochloramine on Selected Riverine Fishes . In: Transactions of the American Fisheries Society . tape 108 , no. 1 , 1979, p. 88-96 , doi : 10.1577 / 1548-8659 (1979) 1082.0.CO; 2 .




