Dichloramine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
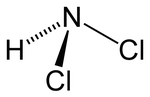
|
|||||||||||||
| General | |||||||||||||
| Surname | Dichloramine | ||||||||||||
| Molecular formula | NHCl 2 | ||||||||||||
| Brief description |
yellow gas |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 85.92 g mol −1 | ||||||||||||
| Physical state |
gaseous |
||||||||||||
| pK s value |
approx. 7 |
||||||||||||
| solubility |
soluble in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Dichloramine is a chemical compound that belongs to the group of chloramines or nitrogen halides .
Occurrence
Dichloramine can be formed by the reaction of chlorine with nitrogen-containing compounds, for example in swimming pools, via monochloramine as an intermediate product.
Extraction and presentation
Dichloramine can be obtained by reacting monochloramine with chlorine or sodium hypochlorite :
Individual evidence
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 699-700.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Water quality of the swimming and bathing pools. Federal Environment Agency , November 13, 2013, accessed June 30, 2017 .
- ^ Reefkeeping.com: Randy Holmes-Farley: Chloramine and the Reef Aquarium .
- ↑ Howard T Dryden: Trichloramine and Asthma in Swimming pools & spas - Problem solved (PDF; 217 kB) from October 1, 2006.
