Clobutinol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
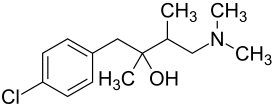
|
||||||||||||||||||||||
| Simplified structural formula - mixture of four stereoisomers | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Clobutinol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 14 H 22 ClNO | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 255.78 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Clobutinol (original trade name : Silomat , Boehringer Ingelheim ) is a medicinal substance that was used as a cough suppressant ( antitussive ). Clobutinol inhibits the cough center in the brain and relieves strong irritable cough ("dry" cough).
In mid-2008, the marketability of all finished medicinal products containing clobutinol ended after side effects on the heart with a risk of life-threatening consequences became known. (see " From market launch to recall ")
Clinical information
application areas
Clobutinol was used for dry, irritating coughs.
unwanted effects
Until it was recalled, clobutinol was considered a very well tolerated and safe drug.
In a not yet fully published study in healthy volunteers , clobutinol led, depending on the dose, to a considerable prolongation of the QT interval in the ECG , which exceeds the currently accepted limit values. A QT prolongation can cause potentially life-threatening cardiac arrhythmias (" torsade arrhythmias "). One case of a torsade arrhythmia has so far been found in the literature. A case of a grand mal seizure after an overdose could theoretically also be associated with the effects on the ECG.
Furthermore, isolated cases of allergic reactions were also known.
Mechanism of action
Clobutinol inhibits the cough center in the elongated medulla ( medulla oblongata ) and thus reduces the frequency and intensity of coughs. In the case of a productive or mucous cough, the desired coughing up of the mucus could be suppressed. Therefore, clobutinol was only recommended for use in dry, irritating coughs.
From market launch to recall
Clobutinol was discovered by Dr. Karl Thomae GmbH (now part of Boehringer Ingelheim) and registered for a patent in 1960/61. In 1961 it was introduced as a finished medicinal product in Germany under the name Silomat .
After the occurrence of a torsade arrhythmia in a young boy became known in 2004, the instructions for use were initially provided with appropriate information, and the German Federal Institute for Drugs and Medical Devices (BfArM) asked the manufacturer to test clobutinol for possible effects on the electrical conduction of the heart to investigate. According to the manufacturer, more than 200 million patients had taken the drug, which is often available without a prescription, for 40 years. The active ingredient was also considered safe among experts.
After submission of the study with volunteers, on August 31, 2007 the BfArM ordered the suspension of approval for all drugs containing clobutinol . The decision of the BfArM was based on a risk-benefit analysis: although the risk of potentially life-threatening side effects is to be assessed as low, other drugs are available for the treatment of the rather mild disease, dry cough. On the same day, Boehringer Ingelheim withdrew all of its own clobutinol-containing drugs from trading worldwide.
In October 2007, the recommendation of the Committee for Medicinal Products for Human Use of the European Medicines Agency to revoke the authorization for all clobutinol-containing medicinal products in the EU followed. A corresponding decision by the European Commission was implemented by the BfArM in June 2008.
After the recall of clobutinol, it was speculated that - statistically speaking - it was established that numerous patients had died of clobutinol. Nobody suspected the cough medicine, which is considered safe, to cause incidents. The risk of QT prolongation with life-threatening consequences was initially assessed as low. The boy who developed the torsade arrhythmia as a result of taking clobutinol survived despite preexisting heart damage.
Boehringer Ingelheim has been selling cough suppressants under the Silomat brand again since November 2008 . Clobutinol has been replaced by the active ingredient pentoxyverine . There is also a drug called Silomat DMP on the market. It contains dextromethorphan as an active ingredient .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Commission decision of 10-I-2008 regarding the authorization (s) of a medicinal product (s) for human use with the active ingredient "clobutinol" in accordance with Article 107 of Directive 2001/83 / EC of the European Parliament and of the Council. Appendix 2. (PDF; 16 kB) European Commission, January 10, 2008; Retrieved September 28, 2008.
- ↑ a b c d e C. Bellocq, R. Wilders, JJ Schott et al: A common antitussive drug, clobutinol, precipitates the long QT syndrome 2. In: Mol Pharmacol . 66, 2004, pp. 1093-1102. PMID 15280442
- ↑ MS Ramirez, AM Rojas, LA Perez, IA Arias, M. Calles, A. Aranguren: Grand mal seizure and clobutinol overdose. In: Vet Hum Toxicol. , 35, 1993, p. 444. PMID 8249270
- ↑ CS Seitz, EB Bröcker, A. Trautmann: Allergy evaluation after emergency treatment: anaphylaxis to the over-the-counter medication clobutinol. In: Emerg Med J. 24, 2007, p. E19. PMID 17351213
- ↑ Dr. Karl Thomae GmbH (1961). US Patent 3121087. Amino-substituted butanols as cough-depressants. Retrieved September 28, 2008.
- ↑ a b c story. 1948-1988 . ( Memento of the original from October 26, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Boehringer Ingelheim GmbH; Retrieved September 28, 2008.
- ↑ a b A. S. Kekulé: Deadly cough syrup. In: Tagesspiegel. September 5, 2007; accessed on March 9, 2015.
- ↑ Clobutinol-containing drugs: BfArM orders suspension of approval. August 31, 2007, accessed February 24, 2016.
- ↑ Medicines containing clobutinol: BfArM orders the approval to be revoked. June 13, 2008.