Cryptophycins
The cryptophycin are a family partly strongly cytotoxic and antibiotic effective macrocyclic depsipeptides .
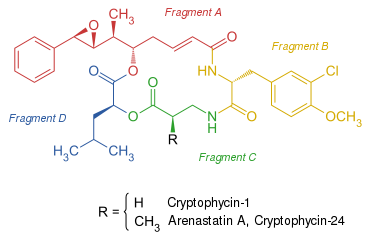
Cryptophycins consist of four building blocks, two amino and two hydroxy acids . One of the hydroxy acid building blocks (fragment A) is of polyketidic origin and, depending on the cryptophycin, has up to four stereogenic centers .
history
Cryptophycins were first described in 1990. R. Schwartz from Merck isolated the substance cryptophycin-1 from cyanobacteria of the genus Nostoc . The compound showed a strong killing effect on yeasts of the genus Cryptococcus , which led to the name.
The work of R. Moore aroused further interest in this class of compounds, who, in addition to purifying the natural product from cyanobacteria of the genus Nostoc, also dealt with the structure elucidation and the potential of these compounds for use in chemotherapy .
I. Kitagawa from the University of Osaka discovered, independently of R. Schwartz and R. Moore, members of the same peptide family in the sea sponge Dysidea arenaria , which he called arenastatins . He was the first to clarify the structure of a representative of this class of compounds. D. Sherman of the University of Michigan clarified the production of cryptophycin in Nostoc on.
properties
Some of the cryptophycins have extraordinarily high cytotoxicity . The IC-50 values reach the low pico molar range and are thus considerably below those of active ingredients with comparable mechanisms of action such as paclitaxel . The effectiveness of cryptophycin-1 remains largely intact in view of drug-resistant tumor cell lines and only decreased by a factor of 6 in the experiment, while it fell 100-fold with vinblastine and even 500-fold with paclitaxel.
As with vinblastine and paclitaxel , the effect of the cryptophycins is based on the binding to tubulin . Cryptophycins thereby disrupt the correct formation of the cytoskeleton and lead to apoptosis . The cause of the effectiveness is the resulting change in the dynamic instability of the microtubules , which means that the correct structure of the spindle apparatus is no longer guaranteed during mitosis . This interrupts cell division and the cell remains in a prometaphase / metaphase-like state before apoptosis is initiated and the cell dies.
As depsipeptides, cryptophycins contain hydrolysis-sensitive ester bonds , and most biologically active cryptophycins also contain a hydrolysis-sensitive epoxide function or a partially stable derivative of the same, so they have to be injected in animal and human experiments to ensure sufficient bioavailability .
Extraction and presentation
The extraction from cyanobacteria of the genus Nostoc and from the sea sponge Dysidea arenaria was only initially important. A large number of chemical syntheses now exist . Attempts at the biotechnological production of cryptophycins have been made, but have so far not been widely used.
use
The Eli Lilly company investigated the suitability of various cryptophcyins for the chemotherapy of cancer diseases in clinical studies of phases 1 and 2 , the results of which were published between 2002 and 2006. To date, there is no drug based on the cryptophycin lead structure .
literature
- RE Schwartz, CF Hirsch, DF Sesin, JE Flor, M. Chartrain, RE Fromtling, GH Harris, MJ Salvatore, JM Liesch, K. Yudin: Pharmaceuticals from Cultured Algae. In: J. Ind. Microbiol. 1990, 5, pp. 113-124.
- C. Sessa, K. Weigang-Kohler, O. Pagani, G. Greim, O. Mor, T. DePas, M. Burgess, I. Weimer, R. Johnson: Phase I And Pharmacological Studies of the Cryptophycin Analogue LY355703 Administered on a Single Intermittent or Weekly Schedule. In: Eur. J. Cancer. 2002, 38, pp. 2388-2396.
- G. D'Agostino, J. DelCampo, B. Mellado, MA Izquierdos, T. Minarik, L. Cirri, L. Marini, JL Perez-Gracia, G. Scambia: A Multicenter Phase II Study of the Cryptophycin Analog LY355703 in Patients with Platinum-Resistant Ovarian Cancer. In: Int. J. Gynecol. Cancer. 2006, 16, pp. 71-76.
- M. Nahrwold, S. Eißler, N. Sewald: Cryptophycins - Highly cytotoxic depsipeptides. highlights, challenges, and recent advances. In: Focus on Peptide - Supplement to Chimica Oggi. 2008, 26, 4, pp. 13-16.
- M. Eggen, GI Georg: The cryptophycins: Their synthesis and anticancer activity. In: Med. Res. Rev. 2002, 22, pp. 85-101.
- E. Hamel, DG Covell: Antimitotic Peptides and Depsipeptides. In: Curr. Med. Chem. 2002, 2, pp. 19-53.
- MA Tius: Synthesis of the cryptophycins. In: Tetrahedron. 2002, 58, pp. 4343-4367.
- RS Al-awar, TH Corbett, JE Ray, L. Polin, JH Kennedy, MMWagner, DC Williams: Biological Evaluation of Cryptophyin-52 Fragment A Analogues: Effect of the Multidrug Resistance ATP Binding Cassette Transporters on Antitumor Activity. In: Mol. Cancer Ther. 2004, 4, pp. 1061-1067.
- BD-M. Chen, A. Nakeff, F. Valeriote: Cellular Uptake of a Novel Cytotoxic Agent, Cryptophyin-52, by Human THP-1 Leukemia Cells and H-125 Lung Tumor Cells. In: Int. J. Cancer 1998. 77, pp. 869-873.
- K. Kerksiek, MR Mejillano, RE Schwartz, GI George, RH Himes: Interaction of Cryptophycin-1 with Tubulin and Microtubules. In: FEBS Lett . 1995, 377, pp. 59-61.
- CD Smith, X. Zhang: Mechanism of Action of Cryptophycin. In: J. Biol. Chem. 1996, 271, pp. 6192-6198.
- C. Shih, BA Teicher: Cryptophycins: A Novel Class of Potent Antimitotic Antitumor Depsipeptides. In: Curr. Pharm. Des. 2001, 7, pp. 1259-1276.
Web links
- Stefan Eißler: Synthesis of cryptophycins for SAR studies. Dissertation, Uni Bielefeld 2008 urn : nbn: de: hbz: 361-13019 .
- Markus Nahrwold: [beta] 2-amino acids as building blocks of functionalized cryptophycin analogues. Dissertation, Uni Bielefeld 2009 urn : nbn: de: hbz: 361-16733 .
Individual evidence
- ↑ C. Weiss, B. Sammet, N. Sewald: Recent approaches for the synthesis of modified cryptophycins. In: Nat Prod Rep . (2013), Volume 30, No. 7, pp. 924-940. doi : 10.1039 / c3np70022d . PMID 23732943 .
- ↑ G. Trimurtulu, I. Ohtani, GML Patterson, RE Moore, TH Corbett, FAVL Demchik: Total Structures of Cryptophycins, Potent Antitumor Depsipeptides from the Blue-Green Alga Nostoc sp. Strain GSV 224. In: J. Am. Chem. Soc. 1994, 116, pp. 4729-4737.
- ^ CD Smith, X. Zhang, S. Mooberry, GM Patterson, R. Moore: Cryptophycin: A New Antimicrotubule Agent Active Against Drug-Resistant Cells. In: Cancer Res. 1994, 54, pp. 3779-3784.
- ↑ M. Kobayashi, M. Kurosu, N. Ohyabu, W. Wang, I. Kitagawa: The Absolute Stereostructure of Arenastatin A, a Potent Cytotoxic Depsipeptide from the Okinawan Marine Sponge Dysidea arenaria. In: Chem. Pharm. Bull. 1994, 42, pp. 2196-2198.
- ↑ D. Panda, RH Himes, RE Moore, L. Wilson, MA Jordan: Mechanism of Action of the Unusually Potent Microtubule Inhibitor Cryptophycin 1. In: Biochemistry 1997. 36, pp. 12948-12953.
- ↑ D. Panda, K. DeLuca, D. Williams, MA Jordan, L. Wilson: Antiproliferative Mechanism of Action of Cryptophycin-52: Kinetic Stabilization of Microtubule Dynamics by High-Affnity Binding to Microtubule Ends. In: Proc. Natl. Acad. Sci. 1998, 95, pp. 9313-9318.
- ↑ SL Mooberry, L. Busquets, G. Tien: Induction of Apoptosis by Cryptophycin 1. In: A New Antimicrotubule Agent, Int. J. Cancer. 1997, 73, pp. 440-448.
- ↑ L. Drew, RL Fine, TN Do, GP Douglas, DP Petrylak: The Novel Antimicrotubule Agent Cryptophycin 52 (LY355703) Induces Apoptosis Via Multiple Pathways in Human Prostate Cancer Cells. In: Clin. Canc. Res. 2002. 8, pp. 3922-3932.
- Jump up ↑ P. Barbier, C. Gregoire, F. Devred, M. Sarrazin, V. Peyrot: In Vitro Effect of Cryptophycin 52 on Microtubule Assembly and Tubulin: Molecular Modeling of the Mechanism of Action of a New Antimitotic Drug. In: Biochemistry 2001. 40, pp. 13510-13519.
- ↑ C. Weiß, T. Bogner, B. Sammet, N. Sewald: Total synthesis and biological evaluation of fluorinated cryptophycins. In: Beilstein J Org Chem. (2012), Volume 8, pp. 2060-2066. doi : 10.3762 / bjoc.8.231 . PMID 23209540 ; PMC 3511040 (free full text).
- ↑ KL Bolduc, SD Larsen, DH Sherman: Efficient, divergent synthesis of cryptophycin unit A analogues. In: Chem Commun (Camb). (2012) PMID 22617820 ; PMC 3494784 (free full text).
- ↑ S. Eissler, A. Stoncius, M. Nahrwold, N. Sewald: The Synthesis of Cryptophycins. In: Synthesis. 2006, pp. 3747-3789.
- ↑ NA Magarvey, ZQ Beck, T. Golakoti, Y. Ding, U. Huber, TK Hemscheidt, D. Abelson, RE Moore, DH Sherman: Biosynthetic Characterization and Chemoenzymatic Assembly of Cryptophycins. Potent Anticancer Agents from Nostoc Cyanobionts. In: ACS Chem. Biol. 2006, 1, pp. 766-779.
- ↑ Y. Ding, CM Rath, KL Bolduc, K. Håkansson, DH Sherman: Chemoenzymatic synthesis of cryptophycin anticancer agents by an ester bond-forming non-ribosomal peptide synthetase module. In: J Am Chem Soc. (2011), Volume 133, No. 37, pp. 14492-14495. doi : 10.1021 / ja204716f . PMID 21823639 ; PMC 3174474 (free full text).
- ↑ JP Stevenson, W. Sun, M. Gallagher, R. Johnson, D. Vaughn, L. Schuchter, K. Algazy, S. Hahn, N. Enas, D. Ellis, D. Thornton, PJ O'Dwyer: Phase I Trial of the Cryptophycin Analogue LY355703 Administered as an Intravenous Infusion on a Day 1 and 8 Schedule Every 21 Days. In: Clin. Canc. Res. 2002, 8, pp. 2524-2529.