Cyclizine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
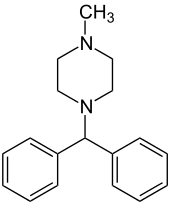
|
|||||||||||||
| General | |||||||||||||
| Surname | Cyclizine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 18 H 22 N 2 | ||||||||||||
| Brief description |
White dust |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| properties | |||||||||||||
| Molar mass | 266.38 g · mol -1 | ||||||||||||
| density |
1.24 g cm −3 (monohydrochloride) |
||||||||||||
| Melting point |
|
||||||||||||
| pK s value |
|
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Cyclizine is a drug from the group of antihistamines of the first generation that was previously used against allergies and motion sickness and is still used in some countries in palliative medicine to treat nausea in children with cancer. Cyclizine can cause poisoning. Illegal use as a substitute drug has also been reported.
Cyclizine is more anti-emetic and less sedating than other antihistamines and was therefore included in the World Health Organization's list of essential medicines for children in 2011. There is currently no approved preparation in Germany.
effect
Cyclizine acts as an antagonist (blocking) on certain acetylcholine and histamine receptors .
Presentation and extraction
The synthesis of cyclizine is achieved by reacting benzhydryl chloride with 1-methylpiperazine .
The active ingredient is used in various salt forms, such as monohydrochloride, dihydrochloride, lactate or tartrate .
Trade names
- Marzine (manufacturer McNeil Denmark ApS ), Valoid, Nausicalm (manufacturer GlaxoSmithKline )
Web links
- Entry on cyclizine at Vetpharm, accessed October 27, 2014.
Individual evidence
- ↑ a b c Entry on cyclizine. In: Römpp Online . Georg Thieme Verlag, accessed on January 10, 2013 .
- ^ V. Bertolasi, PA Borea, G. Gilli, M. Sacerdoti: Cyclizine hydrochloride . In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry . tape 36 , no. 8 , August 15, 1980, p. 1975-1977 , doi : 10.1107 / S0567740880007704 .
- ↑ a b Wolman: in Drug Stand. 25 (1957) 116.
- ^ M. Kuhnert-Brandstätter: Thermomicroscopy in the Analysis of Pharmaceuticals . Pergamon Press 1971, p. 390.
- ↑ a b David W. Newton, Wallace J. Murray, Michael W. Lovell: pKa determination of benzhydrylpiperazine antihistamines in aqueous and aqueous methanol solutions . In: Journal of Pharmaceutical Sciences . tape 71 , no. 12 , 1982, pp. 1363-1366 , doi : 10.1002 / jps.2600711213 .
- ↑ L. Mishack Monene, Colleen Goosen, Jaco C. Breytenbach, Jonathan Hadgraft, Jeanetta du Plessis: Percutaneous absorption of cyclizine and its alkyl analogues . In: European Journal of Pharmaceutical Sciences . tape 24 , no. 2–3 , February 2005, pp. 239-244 , doi : 10.1016 / j.ejps.2004.11.001 .
- ↑ a b Data sheet 1- (DIPHENYLMETHYL) -4-METHYLPIPERAZINE from Sigma-Aldrich , accessed on January 11, 2013 ( PDF ).
- ↑ a b c A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition, Thieme, 2001, ISBN 3-13-115134-X .
- ↑ Boris Zernikow: Palliative care for children, adolescents and young adults. Springer Science & Business Media, 2008, ISBN 978-3-540-48875-0 , p. 198 ( limited preview in Google book search).
- ^ F. Resch, I. Bachner, K. Hruby, K. Lenz: [The intoxication with cyclizin in infancy and adult age (Experiences of a contamination-information-central office) (author's transl)]. In: Clinical Pediatrics. Volume 194, Number 1, January 1982, pp. 42-45, doi: 10.1055 / s-2008-1033768 . PMID 7062687 .
- ↑ Nicholas Seivewright: Community Treatment of Drug Misuse. Cambridge University Press, 2000, ISBN 978-0-521-66562-9 , p. 138 ( limited preview in Google Book Search).
- ↑ Consultation sheet (PDF; 1.3 MB) of the WHO 2008
- ↑ CHEBI: 3994 - cyclizine .
- ↑ Patent US2630435 : Published March 3, 1953 , Applicant: Burroughs Wellcome.
- ↑ KE Hamlin, Arthur W. Weston, Francis E. Fischer, RJ Michaels: Histamine Antagonists. II.1 Unsymmetrical 1,4-Disubstituted Piperazines . In: Journal of the American Chemical Society . tape 71 , no. 8 , August 1, 1949, p. 2731-2734 , doi : 10.1021 / ja01176a038 .
