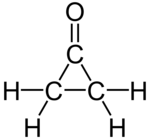
Cyclopropanone
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Cyclopropanone | |||||||||
| Molecular formula | C 3 H 4 O | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 56.06 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cyclopropanone , a derivative of cyclopropane , is the first member of the homologous series of monocyclic ketones ( cycloalkanones ). In its properties - e.g. B. the comparatively low stability - it differs significantly from the higher cycloalkanones.
history
Around 1930, the German chemist Peter Lipp had the idea of synthesizing cyclopropanone from ketene and diazomethane . His motivation is a typical example of the mindset of an organic chemist in the 20th century. However, the conversion in diethyl ether led to cyclobutanone . It was therefore assumed that this was made from the apparently very reactive three-ring ketone, i.e. H. an intermediate stage (intermediate). Lipp and co-workers were then able to isolate the hydrate of cyclopropanone by adding water , which, however, quickly isomerized to propionic acid . With methyl and ethyl alcohol they obtained cyclopropanone hemiacetals.
This pioneering work could be confirmed after about three decades with meanwhile advanced laboratory techniques in the Dutch working group around TJ deBoer . If ketene and diazomethane were allowed to react at −78 ° C in liquefied propane or fluorotrichloromethane / chloroform , the three-ring ketone could be isolated and investigated. Cyclopropanone was found to be stable when stored at the temperature of liquid nitrogen (approx. −195 ° C). When "heating" to 0 ° C., a polymer formed in an exothermic reaction . Even in the gas phase, polymerization occurs on the glass surface.
In the USA, Nicholas Turro and WB Hammond were researching the chemistry of cyclopropanone and especially its methyl derivatives around the same time.
McGee and co-workers synthesized cyclopropanone using a cryochemical technique at −145 ° C from the liquid reactants (ketene and diazomethane) and studied the mass spectrum and the decomposition of the ketone.
properties
Physical Properties
Analysis of a microwave spectrum revealed unequal C – C bonds in the three-membered ring: While the C1 – C2 and C1 – C3 bonds (147.5 pm ) are shortened compared to cyclopropane (151 pm), the bond length is C2 -C3 (157.5 pm) increased. This is in line with MO theoretical considerations and calculations. The valence angle at the carbonyl carbon atom (C1) is only 57 ° 42 'and proves the high tension energy (approx. 188 kJ / mol ) of the molecule. The C = O bond length (119.1 pm) is relatively short. The infrared spectrum shows a C = O stretching vibration of 1816 cm −1 , a value that clearly stands out from that of other ketones.
Numerous MO-theoretical calculations have dealt with the cyclopropanone molecule. It has been postulated that cyclopropanone could be in equilibrium with its valence tautomers allene oxide ( methylenoxirane ) and oxyallyl . Up to now there has been no experimental evidence for this, but there has been evidence for some substituted cyclopropanones (cycloadditions, e.g. on furan ).
Quantum chemical calculations
Stimulated by the problem of valence tautomerism and the analogy to the degenerate rearrangement of methylenecyclopropane (trimethylene methane as intermediate?), Several MO calculations for the system cyclopropanone / oxyallyl / allene oxide were presented, starting with the simple Hückel (HMO) analysis by Burr and Michael Dewar and the theoretical analysis by Roald Hoffmann .
Chemical properties
When cyclopropanone is irradiated in the gas phase, photolysis to carbon monoxide and ethene occurs .
As expected, cyclopropanone is very reactive in solution. Alcohols are added to the carbonyl group very quickly, e.g. B. methanol. Acetic acid forms 1-acetoxycyclopropanol. While the corresponding hemiaminal (1-dimethylaminocyclopropanol) is formed with dimethylamine , methylamine reacts with two molecules of cyclopropanone.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ P. Lipp, J. Buchkremer, H. Seeles: Studies in the Cyclopropan series. Cyclo-propanone. In: Justus Liebig's Annals of Chemistry. 499, 1932, pp. 1-25, doi : 10.1002 / jlac.19324990102 .
- ↑ SE Schaafsma, H. Steinberg, TJ de Boer, The synthesis of cyclopropanone , Recueil des Travaux Chimiques des Pays-Bas 85 , 1170-1172 (1966), doi : 10.1002 / recl.19660851113 .
- ^ NJ Turro, WB Hammond: Cyclopropanones — VIII. In: Tetrahedron. 24, 1968, pp. 6017-6028, doi : 10.1016 / S0040-4020 (01) 90985-8 .
- ^ Nicholas J. Turro: Cyclopropanones. In: Accounts of Chemical Research. 2, 1969, pp. 25-32, doi : 10.1021 / ar50013a004 .
- ↑ EF Rothgery, RJ Holt and HA McGee, Jr., Cryochemical Synthesis and Molecular Energies fo cyclopropanones and Some Related Compounds , J. Amer. Chem. Soc. 97 , 4971 (1975), doi : 10.1021 / ja00850a034 .
- ↑ JM Pochan, JE Baldwin, WH Flygare, J. Am. Chem. Soc. 91 , 1896 (1969).
- ↑ JG Burr, MJS Dewar: The mechanism of the Favorski reaction. In: Journal of the Chemical Society (Resumed). 1954, pp. 1201-1203, doi : 10.1039 / JR9540001201 .
- ^ R. Hoffmann, Trimethylene and the Addition of Methylene to Ethylene , J. Am. Chem. Soc. 90: 1475-1485 (1968).
- ↑ LJ Schaad, BA Hess, Jr., R. Zahradnik, Ab Initio Self-Consistent-Field Study of Favorskii Rearrangement Intermediates, J. Org. Chem. 46 , 1909-1911 (1981).
- ↑ JV Ortiz, Molecular Orbital Theory of Alkylideneoxirane-Cyclopropanone Rearrangements , J. Org. Chem. 48 , 4744-4749 (1983).
- ↑ WJM van Tilborg, H. Steinberg, TJ de Boer, The chemistry of small ring compounds. Part 25. 1-Acetoxycyclopropanol, a convenient precursor for cyclopropanone , Recueil des Travaux Chimiques des Pays-Bas 93 , 287-289 (1974), doi : 10.1002 / recl.19740931106 .
- ↑ WJ M van Tilborg, H. Steinberg, TJ de Boer, The chemistry of small ring compounds. Part 26. The chemistry of 1- (dimethylamino) cyclopropanol , Recueil des Travaux Chimiques des Pays-Bas 93 , 290-293 (1974), doi : 10.1002 / recl.19740931107 .
- ↑ WJM van Tilborg, H. Steinberg, TJ de Boer, The chemistry of small ring compounds. Part 27. Heterocyclic condensation products from cyclopropanone and methylamine , Recueil des Travaux Chimiques des Pays-Bas 93 , 294-297 (1974) doi : 10.1002 / recl.19740931108 .
literature
- Nicholas J. Turro: Cyclopropanones. In: Accounts of Chemical Research. 2, 1969, pp. 25-32, doi : 10.1021 / ar50013a004 .
- Arthur Greenberg, Tyler A. Stevenson in Molecular Structure and Energetics, Vol. 3: "Studies of Organic Molecules" (Edited by Joel F. Liebman and Arthur Greenberg), pp. 195-233, VCH Publishers, Deerfield Beach, 1986.


