O , O- diethyldithiophosphate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
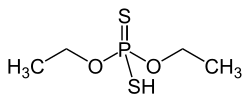
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | O , O- diethyldithiophosphate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 11 O 2 PS 2 | |||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 186.23 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.17 g cm −3 (20 ° C) |
|||||||||||||||
| boiling point |
105-108 (20 hPa) |
|||||||||||||||
| Vapor pressure |
0.66 hPa (57 ° C) mbar (° C) |
|||||||||||||||
| solubility |
soluble in water (330 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.512 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
O , O -diethyldithiophosphate is a chemical compound from the group of thiophosphoric acid esters .
Extraction and presentation
O , O -diethyldithiophosphate can be obtained by reacting ethanol with phosphorus (V) sulfide .
properties
O , O -diethyldithiophosphate is a colorless liquid with an unpleasant odor that is soluble in water.
use
O , O -Diethyldithiophosphat is useful as an intermediate for the preparation of insecticides such as Chlormephos , ethion or phorate used.
Web links
- EPA: IUCLID data set O , O -diethyl dithiophosphate (PDF; 617 kB)
Individual evidence
- ↑ a b c d e f g h i j k data sheet O, O-Diethyldithiophosphat (PDF) from Merck , accessed on June 14, 2012.
- ↑ Patent application EP0036485B1 : Process for converting alcohols and / or phenols with phosphorus pentasulphide. Registered on February 17, 1981 , published on May 6, 1983 , applicant: Höchst, inventor: Werner Krause, Jürgen Grosse, Werner Klose.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . Elsevier, 1996, ISBN 978-0-8155-1401-5 , pp. 362 ( limited preview in Google Book search).



