Ethion
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
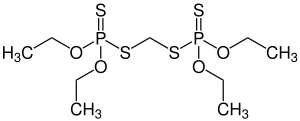
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethion | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 22 O 4 P 2 S 4 | |||||||||||||||
| Brief description |
liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 384.48 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.22 g cm −3 |
|||||||||||||||
| Melting point |
−13 to −12 ° C |
|||||||||||||||
| boiling point |
165 ° C at 0.4 mbar |
|||||||||||||||
| Vapor pressure |
0.0002 Pa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1,549 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.4 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethion is a chemical compound from the group of thiophosphoric acid esters .
Extraction and presentation
Ethion can be prepared by reaction of dibromomethane with the sodium salt of O , O -Diethyldithiophosphorsäure or by condensation of O , O -Diethyldithiophosphorsäure with formaldehyde can be obtained.
properties
Ethion is a colorless to brown odorless liquid in its pure form. The technical product has a strong odor. The compound is stable under normal conditions and in closed vessels, oxidizes slowly in air and hydrolyzes under acidic and basic conditions.
use
Ethion is used as an insecticide and was first marketed by FMC Corporation . It works as a cholinesterase inhibitor .
Admission
Ethion was approved for use in citrus fruits in the United States in the 1950s. In 1998 its use there was restricted.
In the EU countries such as Germany and Austria as well as in Switzerland, no pesticides are permitted that contain ethion as an active ingredient.
Trade names
Ethion was sold under the trade names Ethanox, Ethiol, Hylemox, Nialate, Rhodiacide, Rhodocide, RP-Thion, Tafethion, Vegfru Fosmite, among others.
Web links
- Joint Meeting on Pesticide Residues (JMPR), Monograph for Ethion , accessed December 9, 2014.
Individual evidence
- ↑ a b c d e f g Entry on ethion in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ International Chemical Safety Card (ICSC) for Ethion at the National Institute for Occupational Safety and Health (NIOSH), accessed December 9, 2014.
- ↑ a b Ethion data sheet at Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c d e Entry Ethion at Extoxnet, accessed on December 26, 2017.
- ^ National Research Council: Regulating pesticides: a report . 1980, ISBN 0-309-02946-5 ( page 157 in the Google book search).
- ↑ Entry on Ethion in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 563-12-2 or Ethion ), accessed on November 2, 2015.
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 ( page 352 in the Google book search).
- ^ Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals . Royal Soc of Chemistry, 1999, ISBN 978-0-85404-499-3 ( page 296 in Google book search).
- ↑ EPA: Ethion RED
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Ethion (aka diethion) in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved March 3, 2016.

