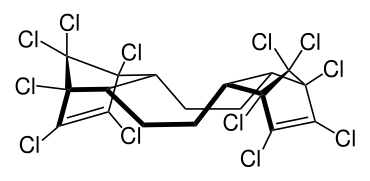
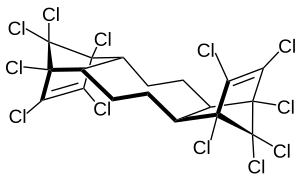
Dechlorane Plus
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Dechlorane Plus | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 12 Cl 12 | |||||||||||||||
| Brief description |
odorless white powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 653.72 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
300 ° C (decomposition) |
|||||||||||||||
| solubility |
slightly soluble in chloroform |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : very persistent and very bioaccumulative ( vPvB ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dechloran Plus (abbreviation DDC-CO ) is a chemical compound that is used as a flame retardant .
Extraction and presentation
DDC-CO is produced by means of the Diels-Alder reaction of two equivalents of hexachlorocyclopentadiene and one equivalent of 1,5-cyclooctadiene . It produced syn - and anti isomer in the ratio of about 1: 3.
use
DDC-CO and other structurally related substances are used as a substitute for Mirex (also called dechlorane ) as a flame retardant. In the electrical an average concentrations of 33 ± 11 was in a 2011 study, ppm found, confirming the presence of non DDC-CO in electronic devices.
Environmental relevance
DDC-CO has been detected in the environment in the air, soil, water, sediment and biota .
Individual evidence
- ↑ a b c d e Entry on Dechloran Plus at Toronto Research Chemicals , accessed on December 27, 2019 ( PDF ).
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on January 19, 2018.
- ↑ Åke Bergman , Andreas Rydén, Robin J. Law, Jacob de Boer, Adrian Covaci, Mehran Alaee, Linda Birnbaum, Myrto Petreas, Martin Rose, Shinichi Sakai, Nele Van den Eede, Ike van der Veen: A novel abbreviation standard for organobromine , organochlorine and organophosphorus flame retardants and some characteristics of the chemicals . In: Environment International . tape 49 , 2012, p. 57–82 , doi : 10.1016 / j.envint.2012.08.003 , PMC 3483428 (free full text).
- ↑ a b Ed Sverko et al .: Dechlorane® plus and related compounds in the environment: a review . Environmental Science & Technology 2011, 45 (12), 5088-5098; doi : 10.1021 / es2003028 .
- ^ J. Gabriel Garcia, Frank R. Fronczek, Mark L. McLaughlin: Tandem reverse-electron-demand diels-alder reactions of 1,5-cyclooctadiene. In: Tetrahedron Letters. 32, 1991, pp. 3289-3292, doi : 10.1016 / S0040-4039 (00) 92688-1 .
- ^ Environmental Health Criteria (EHC) for Mirex , accessed May 4, 2020.
- ^ Ruedi Taverna, Rolf Gloor, Urs Maier, Markus Zennegg, Renato Figi, Edy Birchler: Material flows in Swiss electronic waste . Metals, non-metals, flame retardants and polychlorinated biphenyls in small electrical and electronic devices . Federal Office for the Environment , Bern 2017. Environmental status No. 1717: 164 p.
Web links
- Dechlorane Plus High Production Volume (HPV), Chemical Challenge Program Test Plan, 201-15635 ( Memento from October 23, 2012 in the Internet Archive )