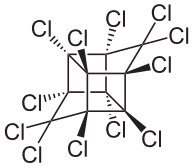
Mirex
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Mirex | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 Cl 12 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 545.55 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
485 ° C (decomposition) |
||||||||||||||||||
| Vapor pressure | |||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Mirex is a cyclic organic chemical compound from the group of chlorinated hydrocarbons that was used as an insecticidal active ingredient - especially against fire ants , leaf cutter ants and termites . Mirex was also used as a flame retardant in plastic and rubber parts, paints and electrical appliances. In electronic waste an average concentration of 4 was in a 2011 study conducted ppm found what the presence of Mirex confirmed in electronic devices.
Mirex is suspected of being carcinogenic. It is potentially liver damaging and affects the immune and hormonal systems. It is very poisonous to crustaceans. The half-life for degradation is about ten years. Under the influence of UV light , it is mainly converted to Photomirex (8-Monohydromirex, C 10 HCl 11 ) and further to 2,8-Dihydromirex (C 10 H 2 Cl 10 ).
Analytical evidence
The chemical-analytical detection in environmental samples, food and animal feed is carried out after suitable sample preparation to separate the matrix and gas chromatographic separation of minor components by high-resolution mass spectrometry techniques such as flight mass spectrometry (Time-Of-Flight mass spectrometry).
Prohibition
In Stockholm Convention of 22 May 2001 for a worldwide ban on the manufacture, sale and use of twelve was persistent organic pollutants ( POP = persistent organic pollutants ) ratified. Under this " dirty dozen " ( dirty dozen ) is also Mirex. On May 17, 2004, after ratification by the 50th accession country, the Convention became global.
Individual evidence
- ↑ a b c Entry on Mirex in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ Entry on Mirex in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on February 1, 2017.
- ↑ Entry on dodecachloropentacyclo [5.2.1.02,6.03,9.05,8] decane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Mirex data sheet at Sigma-Aldrich , accessed on January 31, 2017 ( PDF ).
- ↑ Agency for Toxic Substances and Disease Registry (ATSDR): Mirex and Chlordecone CAS # 2385-85-5 and 143-50-5 (PDF; 223 kB), ToxFAQs, September 1996.
- ↑ Klaus LE Kaiser: Pesticide Report: The rise and fall of mirex , Environ. Sci. Technol. , 1978, 12 (5), 520-528; doi : 10.1021 / es60141a005 .
- ^ Ruedi Taverna, Rolf Gloor, Urs Maier, Markus Zennegg, Renato Figi, Edy Birchler: Material flows in Swiss electronic waste . Metals, non-metals, flame retardants and polychlorinated biphenyls in small electrical and electronic devices . Federal Office for the Environment , Bern 2017. Environmental status No. 1717: 164 p.
- ↑ External identifiers or database links to Photomirex : CAS number: 39801-14-4, PubChem : 91632 , ChemSpider : 82737 , Wikidata : Q81983640 .
- ↑ External identifiers or database links for 2,8-Dihydromirex : CAS number: 57096-48-7, PubChem : 3035224 , ChemSpider : 2299515 , Wikidata : Q95999081 .
- ↑ Health and Safety Guide (HSG) for Mirex , accessed on December 1, 2014.
- ↑ Kelly L. Lambrych, John P. Hassett: Wavelength-dependent photoreactivity of mirex in Lake Ontario , Environ. Sci. Technol., 2006, 40 (3), 858-863; doi : 10.1021 / es0511927 .
- ↑ Eric J. Reiner, Adrienne R. Boden, Tony Chen, Karen A. MacPherson and Alina M. Muscalu: Advances in the Analysis of Persistent Halogenated Organic Compounds . In: LC GC Europe . 23 (2010) 60-70.




