Heptachlor
| General | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surname | Heptachlor | |||||||||||||||
| other names |
1,4,5,6,7,8,8-heptachlor-3a, 4,7,7a-tetrahydro-4,7-methanoindene |
|||||||||||||||
| Molecular formula | C 10 H 5 Cl 7 | |||||||||||||||
| Brief description |
white solid with a camphor- like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 373.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.58 g m −3 (9 ° C) |
|||||||||||||||
| Melting point |
95-96 ° C |
|||||||||||||||
| boiling point |
145 ° C (2 hPa) |
|||||||||||||||
| Vapor pressure |
0.053 Pa (25 ° C) |
|||||||||||||||
| solubility |
poor in water (<1 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 0.05 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Heptachlor is an insecticide that acts as a contact or food poison . It was primarily against soil insects and termites , sometimes against Anopheles -Mücken as malaria -Überträger as well as pesticides used.
In humans, heptachlor leads to liver damage and overstimulation of the central nervous system . It is suspected of causing cancer . The half-life in the soil is up to two years.
Synthesis and composition
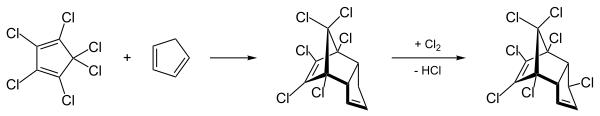
Heptachlor is represented by a reaction of hexachlorocyclopentadiene with cyclopentadiene , in which chlorodene is formed as an intermediate product. This is chlorinated in the dark and with the addition of fuller's earth to make technical heptachlor .
Technical heptachlor consists of a complex mixture of different components. For the most part, the mixture is composed of 72% of the two enantiomers (+) - and (-) - heptachlor, furthermore 18% are trans - chlordane , 2% cis -chlorordane, 2% nonachlor, 1% chlordene, 0.2% Hexachlorbuta-1,3-diene and 10 to 15 other components.
- Main components of heptachlor
Analytical evidence
The chemical-analytical detection in environmental samples, food and animal feed is carried out after suitable sample preparation to separate the matrix and gas chromatographic separation of minor components by high-resolution mass spectrometry techniques such as flight mass spectrometry (Time-of-Flight mass spectrometry).
Heptachlor epoxide
Heptachlor is oxidized biotically and abiotic to heptachlor epoxide. This is more stable than heptachlor and is also broken down more slowly. The abiotic degradation creates a racemic mixture of (-) - and (+) - heptachlorepoxide, while heptachlor is mainly converted into the (+) - enantiomer in the metabolism.
Prohibition
In Stockholm Convention of 22 May 2001 for a worldwide ban on the manufacture, sale and use of twelve was persistent organic pollutants ( POP = persistent organic pollutants ) ratified. That " dirty dozen " includes heptachlor. On May 17, 2004, following ratification by the 50th Accession State, the Convention became globally valid.
No plant protection products containing this active ingredient are permitted in the EU or Switzerland .
In Germany, the limit of 30 ng per liter for heptachlor and its epoxide in drinking water may not be exceeded in accordance with the Drinking Water Ordinance, the maximum amount for (plant-based) food is 10–100 µg / kg.
literature
- Chlordane and Heptachlor , IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.
Web links
- Entry for heptachlor in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire
Individual evidence
- ↑ a b c d e f g h Entry on heptachlor in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ International Chemical Safety Card (ICSC) for heptachlor at the National Institute for Occupational Safety and Health (NIOSH), accessed January 13, 2016.
- ↑ Entry on heptachlor in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 76-44-8 or heptachlor ), accessed on November 2, 2015.
- ^ A b c Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related heptachlor as an undesirable substance in animal feed . In: EFSA Journal . tape 5 , no. 6 , 2007, p. 478 , doi : 10.2903 / j.efsa.2007.478 .
- ↑ Eric J. Reiner, Adrienne R. Boden, Tony Chen, Karen A. MacPherson and Alina M. Muscalu: Advances in the Analysis of Persistent Halogenated Organic Compounds . In: LC GC Europe . 23 (2010) 60-70.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on heptachlor in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.
- ↑ Ordinance on the quality of water for human consumption (Drinking Water Ordinance - TrinkwV 2001 , Annex 2 (to Section 6, Paragraph 2) Part I.
- ↑ Gerhard Eisenbrand (Ed.) And Peter Schreier (Ed.): RÖMPP Lexikon Lebensmittelchemie . 2nd revised edition, 2006; Georg Thieme Verlag; P. 498.