Dibenzyl sulfide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
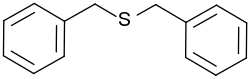
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dibenzyl sulfide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 14 S | ||||||||||||||||||
| Brief description |
white solid with an unpleasant odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 214.33 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.06 g cm −3 |
||||||||||||||||||
| Melting point |
48-50 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dibenzyl sulfide is a chemical compound from the group of thioethers .
Extraction and presentation
Dibenzyl sulfide can be obtained by reacting benzyl chloride with sodium sulfide .
properties
Dibenzyl sulfide is a flammable, hardly flammable, white solid with an unpleasant odor that is practically insoluble in water. At 150 K it has an orthorhombic crystal structure with the space group Pbcn (space group no. 60) .
use
Dibenzyl sulfide is used for organic synthesis.
Individual evidence
- ↑ a b c d e f g h i Entry on dibenzyl sulfide in the GESTIS substance database of the IFA , accessed on January 29, 2020(JavaScript required) .
- ↑ a b c Entry on dibenzyl sulfide in the Hazardous Substances Data Bank , accessed January 30, 2020.
- ^ Christian Hansson: Dibenzyl sulfide at 150 K. In: Acta Crystallographica Section E Structure Reports Online. 62, 2006, p. O2377, doi : 10.1107 / S1600536806017491 .