Diethyl chlorophosphate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
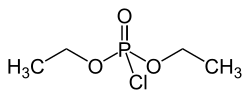
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diethyl chlorophosphate | |||||||||||||||
| other names |
DECP |
|||||||||||||||
| Molecular formula | C 4 H 10 ClO 3 P | |||||||||||||||
| Brief description |
light brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 172.55 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.185 g cm −3 |
|||||||||||||||
| boiling point |
81–82 ° C (16 hPa) |
|||||||||||||||
| Vapor pressure |
0.15 hPa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.416 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diethyl chlorophosphate is a chemical compound of chlorine from the group of organic phosphoric acid esters .
Extraction and presentation
Diethyl chlorophosphate can be obtained by reacting diethyl phosphite with chlorine .
properties
Diethyl chlorophosphate is a flammable, hardly inflammable, light brown liquid with an unpleasant odor that decomposes in water.
use
Diethyl chlorophosphate is used as an intermediate for organic syntheses (e.g. vinyl phosphates).
Individual evidence
- ↑ a b c d e f g h i j k Entry on diethyl chlorophosphate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Data sheet Diethyl chlorophosphate, 97 +% from AlfaAesar, accessed on April 16, 2017 ( PDF )(JavaScript required) .
- ↑ Data sheet Diethyl chlorophosphate, 97% from Sigma-Aldrich , accessed on April 16, 2017 ( PDF ).
- ↑ a b Viviana Beatriz Dorn: Diethyl Chlorophosphate. In: Synlett. 2005, p. 3171, doi : 10.1055 / s-2005-921920 .
- ↑ Richard P. Pohanish: Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens . William Andrew, 2011, ISBN 978-1-4377-7869-4 , pp. 991 ( limited preview in Google Book Search).
- ^ Entry on diethyl chlorophosphate in the Hazardous Substances Data Bank , accessed April 16, 2017.
- ^ Philippe Savignac, Bogdan Iorga: Modern Phosphonate Chemistry . CRC Press, 2016, ISBN 978-0-203-50367-6 , pp. 5 ( limited preview in Google Book search).
