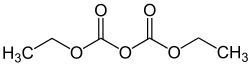
Diethyl dicarbonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diethyl dicarbonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 O 5 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 162.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.12 g cm −3 at 25 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| Refractive index |
1.3960 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diethyl dicarbonate , also called diethyl pyrocarbonate (DEPC), is an ester of pyrocarbonic acid .
use
Diethyl dicarbonate is used in molecular biology to inactivate ribonucleases (RNases). When producing RNase-free water, 0.1% diethyl dicarbonate is autoclaved in water . Unused diethyl dicarbonate hydrolyzes to ethanol and carbon dioxide at these temperatures. Alternatively, some finished solutions can be made up with 0.05% diethyl dicarbonate and autoclaved after 12 h at room temperature, which also inactivates possible RNAse contamination from the reagents.
Until 1973 diethyl dicarbonate was used in the beverage industry for cold pasteurization of fruit juices, wine and beer ( cold sterilization ). Diethyl dicarbonate can form ethyl urethane in aqueous acidic solution and in the presence of ammonium ions (NH 4 + ) . For this reason, the addition of diethyl dicarbonate to beverages was banned in 1973. Dimethyl dicarbonate is used as a secondary active ingredient .
Individual evidence
- ↑ a b c d e Diethyl pyrocarbonate data sheet from Sigma-Aldrich , accessed on March 25, 2011 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-166.
- ↑ Entry on diethyl dicarbonate in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Summers, WC (1970): A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. In: Anal. Biochem. 33 (2): 459-463. PMID 4910776 .
- ↑ Entry on urethane. In: Römpp Online . Georg Thieme Verlag, accessed on March 10, 2014.
