Dilauroyl peroxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
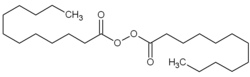
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dilauroyl peroxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 24 H 46 O 4 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 398.63 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.91 g cm −3 |
|||||||||||||||
| Melting point |
55 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (1.1–2.5 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dilauroyl peroxide is a chemical compound from the group of organic peroxides .
Extraction and presentation
Dilauroyl peroxide can be obtained by reacting lauroyl chloride with hydrogen peroxide or sodium peroxide .
properties
Dilauroyl peroxide is a flammable white odorless and tasteless solid that is practically insoluble in water. It begins to decompose when heated above 50 ° C. Calorimetrically a heat of decomposition of -355 kJ · mol was -1 determined. Below the melting point, the decomposition proceeds much more slowly than in the melt. It can react explosively with flammable or reducing substances.
use
Dilauroyl peroxide is used as a bleaching agent and drying agent for fats, oils and waxes and as a polymerization catalyst for vinyl chloride and other compounds. It can also be used as a reducing agent in the Barton-McCombie reaction (deoxygenation of alcohols).
Individual evidence
- ↑ a b c d e f g Entry on dilauroyl peroxide in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Silbert, LS; Swern, D .: Peroxides. VI. Preparation of t-Butyl Peresters and Diacyl Peroxides of Aliphatic Monobasic Acids in J. Am. Chem. Soc. 81 (1959) 2364-2367, doi : 10.1021 / ja01519a023 .
- ↑ Entry on Dilauroyl peroxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Entry on dilauroyl peroxide in the Hazardous Substances Data Bank , accessed on April 21, 2015.
- ↑ a b c Entry on dilauroyl peroxide. In: Römpp Online . Georg Thieme Verlag, accessed on April 21, 2015.
- ↑ Jian-Ming How; Mei-Li You; Yung-Chuan Chu, Chi-Min Shu: Evaluation of thermal hazard for lauroyl peroxide by VSP2 and TAM III in J. Therm. Anal. Calorim. 109 (2012) 1237-1243, doi : 10.1007 / s10973-012-2350-2 .
- ↑ Birgit Janza: New methods in radical heterocyclic synthesis . Cuvillier Verlag, 2006, ISBN 978-3-86727-140-0 , pp. 20 ( limited preview in Google Book search).
