Diphenylarsine chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
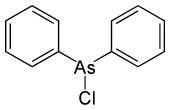
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diphenylarsine chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 10 AsCl | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 264.57 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.42 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
44 ° C |
|||||||||||||||
| boiling point |
Decomposes at 333 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.6332 (56 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diphenylarsane chloride , also known as CLARK 1 ( chlorine - arsenic warfare agent), was presented by Michaelis and La Coste in 1878 and was used as a nasal and throat warfare agent during the First World War . Diphenylarsane chloride is very irritating to the upper respiratory tract and leads to severe nausea .
The aerosols produced by smoldering or nebulization could not be held back by the respiratory protection filters used in gas masks at the time . The affected soldier was forced to take off the mask and was then unprotected from the weapons used at the same time, such as B. exposed to phosgene . The fabric was therefore also called "mask breaker". The monthly production of the mask breaking material CLARK I was 600 tons in the German Reich in 1918.
See also
Individual evidence
- ↑ a b Entry on diphenylarsine chloride. In: Römpp Online . Georg Thieme Verlag, accessed on December 22, 2014.
- ↑ a b c d e Entry on chlorodiphenylarsine in the GESTIS substance database of the IFA , accessed on February 16, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-216.
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ La Coste, W., Michaelis, A. (1878): About mono- and diphenyl arsenic compounds. Ber. 11, 1883-1887.
literature
- Haas, R. et al. (1998): Chemical-analytical investigation of arsenic warfare agents and their metabolites . UWSF - Z Umweltchem Ecotox ; 10, 289-293; PDF (free full text access)

