Entinostat
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
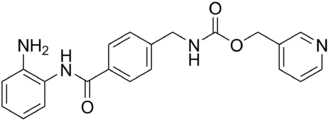
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Entinostat | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 21 H 20 N 4 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 376.4 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Entinostat is an experimental drug from the group of HDAC inhibitors . It has a particular affinity for two class I histone deacetylases (HDAC) , namely HDAC 1 and 3, and should therefore be used as a cytostatic agent . Entinostat is being developed by the pharmaceutical company Syndax and is in phase 2 of the clinical trial .
pharmacology
Mechanism of action
Histone deacetylases (HDACs) are enzymes , the gene expression through deacetylation of lysine residues in histone regulate and are inhibited by Entinostat. This leads to an increased presence of acetylated lysine residues, which no longer have a positive charge and can no longer interact with the negatively charged DNA . The chromatin structure relaxes .
The relaxed chromatin structure enables DNA- binding proteins , such as transcription factors , to access the DNA. This leads to easier gene expression . When therapy with entinostat two objectives:
- Interfering with the expression of genes that play a role in regulating the cell cycle . B. influences cell differentiation or apoptosis .
- Sensitization (medicine) of the tumors to cytostatics , therefore Entinostat should be used as a combination preparation with cytostatics or radiation therapy.
Entinostat has a wide spectrum of activity; it is said to be used against breast cancer , lung cancer , colon cancer , blood cancer and pancreatic cancer . In addition, Entinostat shows a selectivity towards tumor cells and so far no serious side effects .
Pharmacokinetics
Entinostat is effective orally and has a very long plasma half-life of 45 to 100 hours, so one to two doses per week are sufficient .
literature
- T. Suzuki, T. Ando, K. Tsuchiya, N. Fukazawa, A. Saito, Y. Mariko, T. Yamashita, O. Nakanishi: Synthesis and Histone Deacetylase Inhibitory Activity of New Benzamide Derivatives . J. Med. Chem 42 (15), 3001-3003, 1999
- H. Hess-Stumpp, T. Bracker, D. Henderson, O. Politz: MS-275, a potent orally available inhibitor of histone decetylases - The development of an anticancer agent . Int J Biochem Cell Biol. 39, 1388-1405, 2007
- M. Jung: Inhibitor of Histone Decetylase as New Anticancer Agents . Current Medical Chemistry (8), 1505-1511, 2001
- S. Witta, R. Gemmill, F. Hirsch, C. Coldren, K. Hedman, L. Ravdel, B. Helfrich, R. Dziadziusko, D. Chan, M. Sugita, Z. Chan, A. Baron, W. Franklin, H. Drabkin, L. Girand, A. Gazdar, J. Minna, P. Bunn: Restoring E-Cadherin Expression Increases Sensitivity to Epidermal Growth Factor Receptor Inhibitors in Lung Cell Lines . Cancer Research 66 (2), 944-950, 2006
- K. Camphausen, W. Burgan, M. Cerra, K. Oswald, J. Trepel, M. Lee, P.Tofilon. Enhanced Radiation-Induced Cell Killing and Prolongation of γH2AX Foci Expression by the Histone Decetylase Inhibitor MS-275. Cancer Research 64, (316-321 2004)
- S. Park, S. Lee, B. Kim, E. Cho, S. Patel, H. Kang, E. Sausville, O. Nakanishi, J. Trepel, B. Lee, S. Kim: Transcriptional Regulation of the Transforming Growth Factor β Type II Receptor Gene by Histone Acetyltransferase and Deacetylase is Mediated by NF-Y in Human Breast Cancer Cells . The Journal of Biological Chemistry (7), 5168-5174, 2002
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of (pyridin-3-yl) methyl 4- (2-aminophenylcarbamoyl) benzylcarbamate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 21, 2020, is reproduced from a self-classification by the distributor .