Etaconazole
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
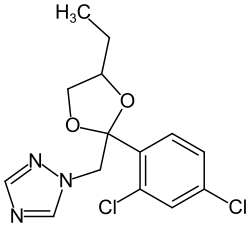
|
||||||||||
| Mixture of stereoisomers - structural formula without stereochemistry | ||||||||||
| General | ||||||||||
| Surname | Etaconazole | |||||||||
| other names |
1 - [(2 RS , 4 RS ; 2 RS , 4 SR ) -2- (2,4-dichlorophenyl) -4-ethyl-1,3-dioxolan-2-ylmethyl] -1 H -1,2,4 -triazole |
|||||||||
| Molecular formula | C 14 H 15 Cl 2 N 3 O 2 | |||||||||
| Brief description |
colorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 328.19 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.4 g cm −3 |
|||||||||
| Melting point |
75-93 ° C |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Etaconazole is a chemical compound belonging to the Conazole group .
Extraction and presentation
Etaconazole can be synthesized starting from a bromination of 2,4-dichloroacetophenone with bromine and subsequent dehydration of the ω-bromo-2,4-dichloroacetophenone formed using 1,2-butanediol and p- toluenesulfonic acid as a catalyst. This is followed by condensation of the 2- (2,4-dichlorophenyl) -2-bromomethyl-4-ethyl-1,3-dioxolane obtained with 1,2,4-triazole to give the end product.
properties
Etaconazole is a colorless solid that is practically insoluble in water. The fungicide consists of four stereoisomers.
use
Etaconazole has been used as a fungicide against powdery mildew on fruits and other crops. It was launched in 1979 but was later replaced by other fungicides. The effect is due to inhibition of sterol - biosynthesis .
Individual evidence
- ↑ a b c d Entry on Etaconazole in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on January 26, 2015.
- ↑ a b c d Datasheet Etaconazole, PESTANAL at Sigma-Aldrich , accessed on October 10, 2016 ( PDF ).
- ↑ a b c S. Gangolli, Royal Society of Chemistry (Great Britain): The Dictionary of Substances and Their Effects: D . Royal Society of Chemistry, 1999, ISBN 978-0-85404-818-2 , pp. 82 ( limited preview in Google Book search).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 978-0-8155-1853-2 , pp. 693 ( limited preview in Google Book search).
- ↑ a b P. Doyle, T. Fujita: Pesticide Chemistry: Human Welfare and Environment: Synthesis and Structure ... Elsevier, 2013, ISBN 978-1-4831-5086-4 , pp. 303 ( limited preview in Google Book search).
