1,2-butanediol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Simplified structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,2-butanediol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 10 O 2 | ||||||||||||||||||
| Brief description |
colorless, viscous liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 90.12 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.01 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−114 ° C |
||||||||||||||||||
| boiling point |
192 ° C |
||||||||||||||||||
| Vapor pressure |
0.1 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.438 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
1,2-Butanediol (according to IUPAC nomenclature : butane-1,2-diol , sometimes also referred to as 1,2-butylene glycol ) is an organochemical compound from the group of dihydric alcohols , or more precisely saturated diols . It is mostly used as a solvent or intermediate in organic syntheses .
Isomers
1,2-Butanediol has one center of chirality ; there are two enantiomers: ( R ) -1,2-butanediol and ( S ) -1,2-butanediol. Without a further specifying addition, a mixture of the two substances is usually meant.
| Isomers of 1,2-butanediol | ||
| Surname | ( R ) -1,2-butanediol | ( S ) -1,2-butanediol |
| Structural formula | 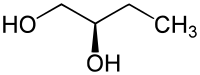 |
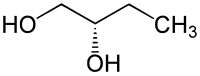
|
| CAS number | 40348-66-1 | 73522-17-5 |
| 584-03-2 (unspec.) | ||
| EC number | 805-781-9 | 805-782-4 |
| 209-527-2 (unspec.) | ||
| ECHA info card | 100.232.775 | 100.232.776 |
| 100.008.663 (unspec.) | ||
| PubChem | 641012 | 6993189 |
| 11429 (unspec.) | ||
| Wikidata | Q27123549 | Q27123550 |
| Q161457 (unspec.) | ||
Extraction and presentation
1,2-butanediol is technically hydration of 1,2-epoxybutane prepared bar without a catalyst at temperatures of 160-220 ° C and pressures of from 10-30.
A 10 to 20-fold molar excess of water is used in order to reduce the formation of polyethers . The selectivities to 1,2-butanediol are 72–92%, depending on the excess water.
The reaction can also be carried out in the presence of a strongly acidic ion exchange resin or smaller amounts of sulfuric acid at temperatures below 160 ° C. and at a slight excess pressure.
It can also be obtained by OsO 4 oxidation of 1- butene in the course of a dihydroxylation .
properties
Physical Properties
Butane-1,2-diol has a relative gas density of 3.1 (density ratio to dry air at the same temperature and pressure ) and a relative density of the steam-air mixture of 1.00 (density ratio to dry air at 20 ° C and normal pressure ). In addition, 1,2-butanediol has a vapor pressure of 0.1 hPa at 20 ° C. The dynamic viscosity is 73 mPa · s at 20 ° C.
Chemical properties
1,2-Butanediol is a flammable, hardly inflammable liquid from the group of alcohols . The substance is miscible with water in all proportions, easily soluble in alcohols and sparingly soluble in esters and ethers . 1,2-Butanediol is insoluble in hydrocarbons . Furthermore, it is considered difficult or very difficult to volatilize. The substance decomposes into formaldehyde when exposed to heat . 1,2-Butanediol can react dangerously with oxidizing agents , reducing agents , acids , acid anhydrides , acid chlorides and chloroformates.
use
1,2-Butanediol is used as a solvent and humectant as well as for the production of epoxy resins , polyamides and polyurethanes . The esters and ethers of 1,2-butanediol are also used as plasticizers .
safety instructions
1,2-Butanediol is a flammable, but hardly inflammable substance. The substance is mainly absorbed through the respiratory tract . Furthermore, a very effective absorption through the skin and the digestive tract is suspected. Acute eye irritation occurs upon ingestion or exposure . Chronic information is not available for humans. A reproductive toxicity and a mutagenic effect could be excluded by tests. Insufficient information is available on carcinogenicity . The ignition temperature is 390 ° C. The substance therefore falls into temperature class T2 and explosion group IIA. The lower explosion point is 92 ° C. With a flash point of 102 ° C, 1,2-butanediol is considered flame-retardant.
Web links
Individual evidence
- ↑ Entry on 1,2-BUTANDIOL in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c d e f g h i j k l Entry on 1,2-butanediol in the GESTIS substance database of the IFA , accessed on August 7, 2019(JavaScript required) .
- ↑ a b Entry on butanediols. In: Römpp Online . Georg Thieme Verlag, accessed on August 7, 2019.
- ↑ Data sheet 1,2-butanediol from Sigma-Aldrich , accessed on August 7, 2019 ( PDF ).
- ↑ Entry on butane-1,2-diol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 7, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c d Heinz Gräfje, Wolfgang Körnig, Hans ‐ Martin Weitz, Wolfgang Reiss, Guido Steffan, Herbert Diehl, Horst Bosche, Kurt Schneider, Heinz Kieczka, Rolf Pinkos: Butanediols, Butenediol, and Butynediol. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., July 23, 2019, p. 11, doi : 10.1002 / 14356007.a04_455.pub2 (section “1,2-Butanediol”).

