Franck-Hertz experiment
The Franck-Hertz experiment is the penultimate link in a three-year series of experiments with which James Franck and Gustav Hertz investigated how much energy is transferred from an electron to an atom when it collides with it. The experiment was carried out in 1914 and, although the two experimenters initially represented a different interpretation, is the first direct evidence of discrete energy levels in atoms, as theoretically required by Niels Bohr in 1913 in Bohr's postulates . The experiment was based on Bohr's model of the atom, which contributed significantly to the advancement of quantum physics until the development of quantum mechanics in 1925 . Franck and Hertz received the Nobel Prize in Physics for this experiment in 1925 .
The experiment measures how much energy remains in the electrons after they have passed through a gas made of mercury atoms by being accelerated by an electric field . The measurements show that the electrons only collide elastically with the atoms after they have passed through an acceleration voltage of less than 4.9 V and transfer practically no energy. Above this threshold they give off 4.9 eV of energy to the atom when they collide . In the last attempt of their series of experiments, Franck and Hertz then demonstrated that the atoms that had absorbed this energy emit light whose photons just have an energy of 4.9 eV. This also confirmed Bohr's second postulate in the experiment. The experiments show that energy is absorbed and released in atoms only in the form of discrete energy packets ( quanta ).
The Franck-Hertz experiment is one of the most impressive examples of quantum physics and at the same time has a relatively simple structure. It is therefore a popular demonstration and practical experiment in physics training.
classification
At the beginning of the 20th century it was known from spectroscopic investigations (e.g. the emission and absorption lines in the case of gases , especially the known Fraunhofer lines and the phenomenon of resonance fluorescence ) and the photoelectric effect of metal surfaces that the energy exchange between light and atoms or also individual electrons only takes place in certain energy packets, the light quanta .
In 1913, Niels Bohr developed the atomic model named after him from the idea that the states of certain fixed energies, the energy levels , also exist in the atom. The line spectrum of hydrogen , which has been known for a long time, could be explained with an additional assumption as to which states an electron can assume in the atom . Accordingly, atoms release or absorb energy by changing from one of the possible states to another and, according to quantum physics, emit or absorb a light quantum whose energy content is given by the energy difference between the two states. The Bohr frequency condition, which corresponds to the formula for the light quanta established by Albert Einstein in 1905, gives the wavelength of the light quantum through the change in energy of the atom:
- .
In it is
- the energy of the initial state,
- the energy of the final state,
- the speed of light ,
- the frequency of the spectral line and
- the Planck constant .
In that the atom receives its energy not from a light quantum but from an inelastic electron impact, the Franck-Hertz experiment confirmed the discrete energy levels of the atom.
Franck and Hertz, however, did not carry out their experiments to check Bohr's model of the atom, as this was only briefly known to them at the time. They wanted to use their apparatus to measure ionization energies of monatomic gases because, according to the theory that was widespread at the time, ionization is the only form in which atoms can absorb energy (apart from the kinetic energy of the entire atom), and because the then generally accepted theory of electrical discharges by JS Townsend failed with monatomic gases. They saw the emergence of the light quanta, the energy of which exactly matched the energy loss of the colliding electrons, as a result of the recombination of the mercury ion with an electron, because this releases exactly the ionization energy. Until the presentation of Bohr's postulates, this was also the generally accepted interpretation. Franck and Hertz reiterated their position that 4.9 eV was the ionization energy, even in 1916, after Bohr's atomic model had already largely gained acceptance and their experiment was generally viewed as a direct confirmation of Bohr's postulates. The convincing experimental proof that mercury is not ionized at an energy input of 4.9 eV, but only at 11.4 eV, was actually only provided in 1917 by Davis and Goucher, and only then did Franck and Hertz agree with the interpretation (as shown in the introduction) to, which had now finally turned out to be the right one.
Franck-Hertz experiment
Experimental set-up
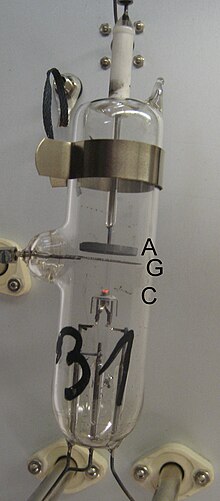
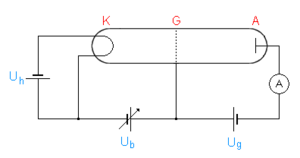
A glass bulb contains a gas (mostly mercury vapor , but neon is also common) at low pressure, typically in the range of 10 to 20 mbar. At one end there is a hot cathode K , which is heated by the power source U h . The grid G at a distance of cm is at a positive potential with respect to the cathode due to the controllable positive voltage U b (in the range of a few V). The collecting electrode A , on which the current is measured, is located directly behind the grid and opposite it is at a slightly negative potential U g of about 1 V.
The purpose of this arrangement is to accelerate electrons between K and G and to let them collide with the mercury atoms. The weak opposing field between G and A then excludes all electrons from the current measurement that do not have a certain minimum energy when passing through G ( opposing field method ).
The electrons emitted by the cathode are accelerated and reach their highest speed immediately in front of the grid. The electrons that land on the grid are transported back to the cathode by the power source U b . The others pass the grid and are slowed down by the weak electric field between the grid and the collecting electrode A. Low-energy electrons cannot overcome the opposing field and ultimately land on the grid. Only the electrons with sufficient energy hit A and are measured on the way back with the help of a sensitive ammeter .
Implementation and observation
If the acceleration voltage is increased from U b = 0 above the value of U g , the measured current values initially begin to increase (area (1) in the figure). From a certain voltage value (depending on the gas filling) the current drops (2), reaches a minimum value and then increases again (3). At around twice the value of the voltage at which the current first drops, it drops again (4) and then rises again. This is repeated approximately periodically, with the current increasing each time to a higher value. (In contrast to many simplified representations, the distances between the maxima and minima are not entirely constant (see below).
When testing with neon, when the first minimum is reached, one sees a glowing layer in front of the grid, which moves towards the cathode as the voltage increases. With each new minimum, another luminous layer is created. This cannot be observed with mercury because the resulting radiation is in the UV range.
The total current through the tube, i.e. the cathode current or the sum of the grid and collector current, shows no such periodic changes, but increases with increasing voltage proportionally to U b 3/2 ( Schottky equation ), as in a tube diode . Unlike in vacuum tubes, however , the current does not saturate at high voltage, but increases suddenly and sharply from a certain ignition voltage (for mercury here around 40 V) due to the gas discharge . In order to avoid damaging the tube, the current is limited in the experimental setup by a suitable resistor in series with the cathode.
Explanation
At low voltages, the current increases with the voltage because the accelerating field becomes stronger and (as in every vacuum tube) more electrons can be sucked out of the space charge zone around the cathode. The drastic drop in current strength when the voltage exceeds a threshold value shows that many electrons have lost energy on their way through the gas, so that they can no longer get through to the collector because of the opposing field. This is explained by the fact that the electrons, as soon as they reach a certain kinetic energy (for mercury approx. 4.9 eV, corresponds to the transition from - to - level), can release this energy when they collide with an atom, i.e. they collide inelastically. If the energy is lower, the atom (as a whole) can only make elastic collisions with the electron, in which practically no energy is transferred due to the large mass difference. In the case of an inelastic collision, the hit atom is excited; that is, the transmitted energy does not appear as its center of gravity. In Bohr's atomic model, the energy is transferred to a single shell electron by raising it to a higher energy level. Since this state is unstable, the electron falls back to its earlier state a short time later (order of magnitude ) emitting a light quantum .
If the electron has kinetic energy just above 4.9 eV before the inelastic collision, it does not have enough energy afterwards to overcome the decelerating opposing field. In the minimum, however, the measured current does not drop to zero, because only some of the electrons collide inelastically with the atoms. There are always electrons that reach the necessary energy (shortly before the grid), but then no longer find a collision partner because of the short path to the grid. In addition, the UV radiation emitted by the excited atoms can release electrons at various points on the tube by photo- effect, which are attracted by the collector electrode and contribute to the measured current. Franck and Hertz, however, erroneously assumed in their original interpretation that the mercury atom is ionized at 4.9 eV and that this minimum value of the current is based on the (positive) ions that now migrate to the cathode. The idea of excited states of neutral atoms introduced by Bohr was still alien to them.
When the acceleration voltage increases further, the zone where the electrons first gained the necessary kinetic energy moves closer to the cathode. Therefore, after losing energy, the electrons are accelerated a little again, so that the number of electrons that overcome the braking voltage increases again (3). This applies until the accelerating voltage is so great that the electrons pick up 4.9 eV again after the first inelastic collision and can collide inelastically a second time (4). Then there are two zones of excited mercury atoms, one halfway to the grid and one just in front of it.
The light emitted by the mercury atoms (the quantum energy 4.9 eV) has a wavelength of approx. 253 nm, however, in the ultraviolet range and is therefore not visible. However, there are further levels at 8 eV which, after the excitation, initially change to the 4.9 eV level and emit a visible green light quantum. The separate luminous zones can be clearly seen with a neon filling if the acceleration voltage is several times sufficient to lift an electron from the fully occupied 2p level to one of the higher 3p levels between 18.4 eV and 19.0 eV. Because the Ne atom excited in this way first loses its excitation energy in a small step to the 3s level, which is approx. 2.5 eV lower, which is why visible orange-yellow light is created.
A more precise interpretation of the experiment, which was only published at the end of the 20th century, takes into account space charge effects and the fact that the electrons do not form a directed beam, but are deflected in all directions (including back) due to the numerous elastic collisions. For a complete explanation, different levels with their different stimulation probabilities must also be taken into account. As a result, for example, the lowest excitation level is observed neither with neon nor with mercury, but a higher one.
Extensions of the experiment
Improved resolution with electrical "magnifying glass"
In the simple circuit of the previous chapter, the acceleration and reaction path of the electrons coincide. By introducing a second grid, it is possible to separate both areas and to detect higher energy levels with a higher energy resolution.
For this purpose, the distance between the cathode and the grid 1 is chosen to be very small, so that the electrons can hardly perform inelastic collisions with gas atoms, while they are accelerated by U G1 (approx. 10 V) almost to the energy required for higher levels. Between grid 1 and grid 2 they are accelerated at a much greater distance by a considerably lower voltage ΔU (of the order of magnitude 0.1 V), as a result of which their speed increases only gradually. As in the original apparatus (above), inelastic collisions of the electrons are demonstrated by the fact that they cannot overcome the subsequent counter-voltage between grid 2 and plate A. The energy threshold to the inelastic collision can thus be observed much more precisely.
Other gas fillings
In order to avoid the use of the poisonous mercury and for didactic reasons, the experiment is carried out with the gas neon, especially in school practicals. Here the excitation energies are higher, they are between 18.4 eV and 19.0 eV - light emission of this energy would not be in the visible range. The de-excitation of the excited neon atoms also takes place via intermediate states with energies in the range between 16.6 eV and 16.9 eV. Therefore, photons in the energy range of 2 eV also arise, which leads to red-orange light emission. If the voltage corresponds to a multiple of the excitation voltage, a corresponding number of adjacent luminous areas can be seen in the tube.
Web links
- LP - The Franck-Hertz-Experiment on the website of the University of Göttingen (incl. Sketches, photos, videos and references)
- Animation at student level ( LEIFI )
- Interactive experiment on the Kansas State University website (English, requires Adobe Shockwave )
Individual evidence
- ↑ J. Franck and G. Hertz: About collisions between electrons and molecules of mercury vapor and the ionization voltage of the same . In: Verh. Dtsch. Phys. Ges. Band 16 , 1914, pp. 457-467 , doi : 10.1002 / phbl.19670230702 . (Excerpts online on LEIFI -Physik)
- ↑ Bergen Davis and FS Goucher: Ionization and Excitation of Radiation by Electron Impact in Mercury Vapor and Hydrogen , In: Phys. Rev. Vol. 10 (1917), pp. 101-115
- ↑ G. Rapior, K. Sengstock, V. Baev: New features of the Franck-Hertz experiment . In: Amer. J. Phys. No. 74 , 2006, pp. 423-428 , doi : 10.1119 / 1.2174033 . ( PDF file; 117 kB; (English) ( Memento of the original from April 13, 2014 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. )
- ^ RE Robson, B. Li and RD White: Spatially periodic structures in electron swarms and the Franck-Hertz experiment . In: J. Phys. B: At. Mol. Opt. Phys . No. 33 , 2000, pp. 507 , doi : 10.1088 / 0953-4075 / 33/3/318 .
- ^ RE Robson, M. Hildebrandt and RD White: A cornerstone of atomic physics . In: Physics Journal . No. 3 , 2014, p. 43 . ( PDF file ( Memento of the original from December 8, 2014 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. )
- ↑ Term scheme in Figure FH.2 (PDF; 171 kB)