Fraxetin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
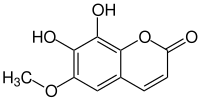
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Fraxetin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 8 O 5 | |||||||||||||||
| Brief description |
yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 208.17 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
230-231 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Fraxetin is a coumarin derivative that, like its glucoside Fraxin, is found in the bark of some tree species - e.g. B. the Chinese flower ash - occurs. Just like the coumarin derivatives esculetin or scopoletin , which are also found in ash bark, it plays an important role in the effectiveness of some uses from traditional Chinese medicine .
effect
Fraxetin is an antioxidant . Among other things, it has been shown that the antioxidant effect gives Fraxetin a neuroprotective effect . In experiments with human nerve cells, z. It has been shown, for example, that Fraxetin reduces the neurodegenerative effects of Rotenone . In fly experiments it could be shown that Fraxetin leads to a reduction in oxidative stress . This effect is based on the one hand on the reaction of Fraxetin itself with reactive oxygen species and on the other hand on the increased formation of glutathione induced by Fraxetin .
synthesis
In addition to obtaining fraxetin by extraction from natural sources such as ash bark, fraxetin can also be obtained by a total synthesis . 2,3,4-trimethoxybenzaldehyde is used as the starting material . This is first reacted with boron trichloride to form 2,3-dihydroxy-4-methoxybenzaldehyde. The developed thereby free hydroxy groups are then, by reaction with isopropyl bromide and Hunig's base with isopropyl - protecting groups provided. Subsequent Baeyer-Villiger oxidation with meta- chloroperbenzoic acid converts the benzaldehyde into a phenol via the corresponding methyl ester . Using coupling reagents such as dicyclohexylcarbodiimide , the phenol is reacted with propiolic acid to form a propiolic acid ester. The triple bond introduced as a result, catalyzed by gold (I) compounds, can react with the C5 carbon of the original starting material and (isopropyl-protected) fraxetin is obtained.
Individual evidence
- ↑ a b c d data sheet 7,8-dihydroxy-6-methoxycoumarin from Sigma-Aldrich , accessed on February 8, 2017 ( PDF ).
- ↑ Ma, Z., Zhao, Z .: Studies on chemical constituents from stem barks of Fraxinus paxiana . In: China J. Chin. Mater. Med. Band 33 , 2008, p. 1990-1993 (English).
- ^ MF Molina-Jiménez, MI Sánchez-Reus., M. Cascales, D. Andrés, J. Benedí: Effect of fraxetin on antioxidant defense and stress proteins in human neuroblastoma cell model of redone neurotoxicity. Comparative study with myricetin and N-acetylcysteine. In: Toxicology and Applied Pharmacology . Volume 209, Number 3, 2005, pp. 214-225, PMID 15904944 .
- ↑ B. Fernández-Puntero, I. Barroso, I. Iglesias, J. Benedí, A. Villar: Antioxidant Activity of Fraxetin: In Vivo and Ex Vivo Parameters in Normal Situation versus Induced Stress. In: Biological and Pharmaceutical Bulletin . Volume 27, Number 7, 2001, pp. 777-784, PMID 11456117 .
- ↑ A. Cervi, P. Aillard, N. Hazeri †, L. Petit, CLL Chai, AC Willis, MG Banwell: Total Syntheses of the Coumarin-Containing Natural Products Pimpinellin and Fraxetin Using Au (I) -Catalyzed Intramolecular Hydroarylation (IMHA ) Chemistry In: Journal of Organic Chemistry . Volume 78, number 19, 2013, pp. 9876-9882, doi: 10.1021 / jo401583q .
