Corilagin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
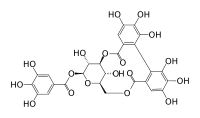
|
|||||||||||||
| General | |||||||||||||
| Surname | Corilagin | ||||||||||||
| Molecular formula | C 27 H 22 O 18 | ||||||||||||
| Brief description |
white solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 634.45 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
208 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Corilagin is a natural substance and belongs to the tannins , a group of tannins of which it is the most important representative. It is a typical representative of the Ellagen tannins.
history
Corilagin was first isolated in 1951 from Dividivi extracts and from Caesalpinia coriaria . In traditional Chinese medicine , extracts of the plant Phyllanthus urinaria , which contain corilagin, were administered as medicinal products due to their antiviral and antibacterial properties.
Extraction and presentation
Corilagin can be obtained from the roots of Euphorbia fisheriana (a milkweed family ) , the pomegranate ( Punica granatum ) or the dried fruits of Terminalia chebula (Chebulian myrobalans ).
The collected plant parts are first crushed and placed in water. The hydrophilic components dissolve in the water . The aqueous solution is then treated with a mixture of diethyl ether and ethanol (in a ratio of 4 to 1) in order to dissolve out the corilagin.
properties
Corilagin forms long, colorless, less characteristic needles that decompose (air-dry) at a little over 200 ° C. It dissolves easily in acetone, methanol and ethanol, difficultly in glacial acetic acid and water, very difficultly in ethyl acetate and is practically insoluble in benzene and ether. It crystallizes from water as a trihydrate and is hygroscopic as an anhydrate.
It belongs to the groups of polyphenols and ellagitannins . Corilagin can be hydrolyzed by diluted sulfuric acid and heating for several days (100 ° C) , whereby gallic acid is split off first and then it decomposes into glucose , ellagic acid and gallic acid.
Biological importance
Corilagin inhibits adrenaline- induced lipolysis in fat cells isolated from rats .
Individual evidence
- ↑ a b c d data sheet Corilagin, analytical standard at Sigma-Aldrich , accessed on May 29, 2016 ( PDF ).
- ↑ Shmuel Yannai: Dictionary of Food Compounds with CD-ROM, Second Edition . CRC Press, 2012, ISBN 978-1-4200-8352-1 , pp. 294 ( limited preview in Google Book search).
- ↑ The Metabolism of Secondary Plant Products / The Metabolism of Secondary Plant Products . Springer Science & Business Media, 2013, ISBN 978-3-662-26784-4 , p. 365 ( limited preview in Google Book Search).
- ^ O. Th. Schmidt, R. Lademann (1951): Liebigs Ann. Chem. , 571, pp. 232-237.
- ^ O. Th. Schmidt, DM Schmidt (1952): Liebigs Ann. Chem. , 578, 25-30.
- ↑ P. Grunwald (1998): Nachr. Chem. Tech. Lab. , 46, pp. 853-857.
- ↑ S.-H. Lee, T. Tanaka, G.-I. Nonaka, I. Nishioka, B. Zhang (1991): Phytochemistry , 30, pp. 1251-1253.
- ↑ MA Nawwar, SAM Hussein, I. Merfort: (1994): Phytochemistry , 36, pp. 793-798.
- ^ O. Th Schmidt, J. Schulz, H. Fiesser (1967): Liebigs Ann. Chem ., 706, pp. 187-197.
- ↑ K. Paech, MV Tracey: Modern Methods of Plant Analysis / Modern Methods of Plant Analysis . Springer Science & Business Media, 2012, ISBN 978-3-642-64958-5 , p. 546 ( limited preview in Google Book search).
- ↑ The components of food . Springer-Verlag, 2013, ISBN 978-3-642-46011-1 , p. 561 ( limited preview in Google Book search).
- ↑ Y. Kimura, H. Okuda, T. Okuda, T. Yoshida, T. Hatano, S. Arichi (1983): Chem Pharm Bull ., 31, pp. 2497-2500.
