Gingerol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
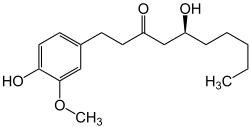
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Gingerol | ||||||||||||||||||
| other names |
( S ) -5-Hydroxy-1- (4-hydroxy-3-methoxy-phenyl) -decan-3-one ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 17 H 26 O 4 | ||||||||||||||||||
| Brief description |
yellowish, odorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 294.39 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Gingerol is an important flavor component of ginger rhizome ( Zingiber officinale ). Gingerol is the main cause of the pungent taste of ginger.
Related substances
So far, six pungent substances have been identified in ginger. Further ingredients with structures similar to the pungent substances are 6-, 8-, 10-gingerol and 6-, 8-, 10-shogaol. 6-Shogaol is a breakdown product of 6-Gingerol, whereby Gingerol loses a hydroxyl group . Zingiberen and zingiberol are also contained in ginger and are important for the taste .
Analytics
For the reliable qualitative and quantitative determination of gingerol in various test materials, the coupling of HPLC with mass spectrometry is suitable after adequate sample preparation .
effect
On the one hand, it is believed that gingerols, especially shogaols, act on the vanilloid receptor and thus trigger an anti-inflammatory reaction. It could be shown that the pungent substances (gingerol, shogaol) have a specific influence on the interleukin / cytokine pattern . The interleukin / cytokine pattern can be used to see different reactions to a disease.
It could also be shown that the pungent substances influence the iNOS (inducable NO synthase ), which may have an impact on the smooth muscles and thus cause a constriction or dilation of the vessels .
Individual evidence
- ↑ Data gingerol (PDF) at Carl Roth , accessed on 14 December of 2010.
- ↑ a b c d data sheet 6-Gingerol from Sigma-Aldrich , accessed on April 3, 2011 ( PDF ).
- ↑ Entry on spicy taste. In: Römpp Online . Georg Thieme Verlag, accessed on July 19, 2014.
- ^ Y. Tao, W. Li, W. Liang, RB Van Breemen: Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. In: J Agric Food Chem. 57 (21), Nov 11, 2009, pp. 10014-10021. PMID 19817455 .
- ↑ C. Schoenknecht, G. Andersen, P. Schieberle: A novel method for the quantitation of gingerol glucuronides in human plasma or urine based on stable isotope dilution assays. In: J Chromatogr B Analyt Technol Biomed Life Sci. 1036-1037, Nov 15, 2016, pp. 1-9. PMID 27700987 .
- ↑ S. Lee, C. Khoo, CW Halstead, T. Huynh, A. Bensoussan: Liquid chromatographic determination of 6-, 8-, 10-gingerol, and 6-shogaol in ginger (Zingiber officinale) as the raw herb and dried aqueous extract. In: J AOAC Int. 90 (5), Sep-Oct 2007, pp. 1219-1226. PMID 17955965 .
- ↑ Tzung-Yan Leea, Ko-Chen Leeb, Shih-Yuan Chena, Hen-Hong Chang: 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. In: Biochemical and Biophysical Research Communications. Volume 382, No. 1, April 24, 2009, pp. 134-139.

