Graphite fluoride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
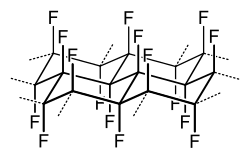
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Graphite fluoride | |||||||||||||||
| Molecular formula | CF x | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 31.01 g mol −1 for x = 1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
(−178.029 x + 4.519) kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Graphite fluoride , CF x is a non- stoichiometric , solid fluorocarbon compound in which x can assume values between 0 and 1.24. Depending on the fluorine content, graphite fluoride appears black ( x ≤ 0.9), brown-gray (0.9 < x ≤ 1.0) or cream-white ( x > 1.0). Graphite fluoride was first synthesized in the 1930s by Otto Ruff and Bretschneider by reacting activated carbon or graphite with fluorine at elevated temperatures.
presentation
Graphite fluoride can be produced by the reaction of ordered carbon , e.g. B. graphite, but also of disordered substrates after heat treatment such. B. pyrolysis coke and even flame soot with fluorine in the temperature range of 400 to 700 ° C are produced. In principle, a highly ordered material such as graphite always provides a higher proportion of graphite fluoride than z. B. a "graphitized" flame black, in which the proportion of volatile fluorocarbons, such as. B. tetrafluoromethane , CF 4 dominates. At higher temperatures (from 700 ° C) only volatile fluorocarbon compounds are formed.
structure
When the graphite is fluorinated, its layer structure is basically retained. However, depending on the type of preparation and composition of the graphite fluoride, the distance between the carbon layers is widened significantly by the formation of the tertiary C – F units and their strong electrostatic repulsion from 335 pm for graphite to up to 580 - 615 pm. The structure of the layers is, however, turbostratic , i.e. H. Layers lying on top of one another are parallel, but do not have any preferred orientation to one another. The individual C 6 F 6 elements of the layers are in a chair conformation, each with axially positioned fluorine atoms, as shown in the structural formula above. In contrast to graphite fluoride, which is a fluorocarbon compound with covalent CF bonds, the reaction of fluorine with e.g. B. carbon at low temperatures, various graphite-fluorine intercalation compounds of the general composition, C y F with y = 2-10, are generated.
properties
In contrast to the anisotropic electrical conductor graphite, its reaction product graphite fluoride, with the ideal stoichiometry CF, is an insulator . If the degree of fluorination is below x = 0.9, graphite fluoride conducts the electrical current just like graphite. Graphite fluoride is a superhydrophobic material and shows a contact angle of 143 ° with water, while polytetrafluoroethylene provides a contact angle of 109 ° for comparison. Graphite fluoride with stoichiometries between x = 0.61 - 1.12 shows excellent lubricating properties, especially in the high temperature range, which neither molybdenum sulfide nor graphite can achieve. Graphite fluoride decomposes at temperatures above 600 ° C, essentially with dismutation, to form difluorocarbene, tetrafluoroethene and expandable graphite.
use
Graphite fluoride is mainly used today as a cathodic depolarizer in the lithium-carbon monofluoride battery . It accelerates the burnout of boron in solid propellant compositions by effectively removing the oxide layer to form carbon monoxide and boron trifluoride. In addition, it is a very high-energy oxidizing agent in infrared decoys and air-breathing drives. During the exothermic reaction of graphite fluoride with reducing agents, nanostructured reaction products such as e.g. B. SiC fibers and carbon nanotubes formed. Graphite fluoride dispersed in hot sulfolane (50 ° C) yields graphene fluoride through ultrasound-induced exfoliation .
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of carbon fluoride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on May 26, 2020, is reproduced from a self-classification by the distributor .
- ↑ Graphite fluoride data sheet from Sigma-Aldrich , accessed on June 13, 2011 ( PDF ).
- ↑ a b c d e P. Kamarchik Jr., JL Margrave, Poly (carbon monofluoride): A Solid, Layered Fluorocarbon, Acc. Chem. Res. , 11 , 1978 , 296. doi : 10.1021 / ar50128a002
- ↑ O. Ruff, O. Bretschneider, The reaction products of the various carbon forms with fluorine II (carbon monofluoride), Z. anorg. u. allg. Chem. , 217, 1934 , 1. doi : 10.1002 / zaac.19342170102
- ↑ a b c T. Nakajima., N. Watanabe, Graphite Fluorides and Carbon-Fluorine Compounds , CRC Press, Boca Raton, 1990
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ MS Dresselhaus, M. Endo, J.-P. Issi, Physical Properties of Fluorine- and Fluoride-Graphite Intercalation Compounds, in T. Nakajima (Ed.), Fluorine-Carbon and Fluoride Carbon Materials , Marcel Dekker, New York, 1995 , 95
- ↑ N. Watanabe, S. Koyama, H. Imoto, Thermal Decomposition of Graphite Fluoride. I. Decomposition Products of Graphite Fluoride, (CF) n in a Vacuum, Bull. Chem. Soc. Jpn. , 53 1980 , 2731. doi : 10.1246 / bcsj.53.2731
- ↑ Gong Chen, Zhongning Shi, Jiangyu Yu, Zhaowen Wang, Junli Xu, Bingliang Gao, Xianwei Hua: Kinetic analysis of the non-isothermal decomposition of carbon monofluoride, Thermochim. Acta , 589 , 2014 , 63-69, doi : 10.1016 / j.tca.2014.05.002
- ↑ T.-K. Liu, I.-M. Shyu, Y.-S. Hsia, Effect of Fluorinated Graphite on Combustion of Boron and Boron-Based Fuel-Rich Propellants, J. Propul. Power , 12 , 1996 , 26. doi : 10.2514 / 3.23986
- ↑ E.-C. Koch, Pyrotechnic composition for producing IR radiation US Patent 6635130, 2003 . US patent 6635130
- ↑ E.-C. Koch, Metal / Fluorocarbon Pyrolants: VI. Combustion behavior and Radiation Properties of Magnesium / Poly (carbon Monofluoride) Pyrolant, Propellants Explos. Pyrotech. , 30 , 2005 , 209. doi : 10.1002 / prep.200500007
- ↑ JL Fields, Combustible Compositions for Air Augmented Rocket Engines US Patent 6736912, 2004 , 2731. US Patent 6736912
- ↑ S: Cudzilo, M. Szala, A. Huczko, M. Bystrzejewski, Combustion Reactions of Poly (Carbon Monofluoride), (CF) n , with Different Reductants and Characterization of the Products, Propellants Explos. Pyrotech. , 32 , 2007 , 149. doi : 10.1002 / prep.200700015
- ↑ E.-C. Koch, Process for the production of carbon allotropes and their intercalates or endohedral compounds , DE Patent 10122750B4 2008
- ↑ Radek Zborˇil, Frantisˇek Karlicky´, Athanasios B. Bourlinos, Theodore A. Steriotis, Athanasios K. Stubos, Vasilios Georgakilas, Klára Sˇafárˇová, Dalibor Jancˇík, Christos Trapalis, and Michal Otyepka, Graphene Fluoride and its Chemical Graphene Stoichative Conversion to Graphene, Small, 6: 2885-2891. doi : 10.1002 / smll.201001401
swell
- Erwin Riedel: Inorganische Chemie , 6th edition, de Gruyter, Berlin 2004, p. 516, ISBN 3-11-018168-1 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 846.

