Hall-Héroult trial
The Hall-Héroult process is a process for producing pure aluminum from aluminum oxide in an aluminum smelter . The starting material aluminum oxide obtained from the Bayer process is dissolved in cryolite and the pure aluminum is then produced using fused- salt electrolysis .
history
In 1886 Charles Martin Hall and Paul Héroult invented this process independently of one another at about the same time. This process is still used in industry today, with some improvements.
Procedure
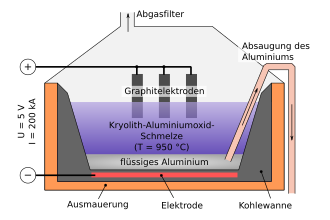
Before the actual electrolysis step , the aluminum oxide (melting temperature 2045 ° C ) is mixed with cryolite (Na 3 [AlF 6 ]) in order to lower the melting temperature. The resulting mixture, which consists of 80 to 90 percent cryolite, has a melting temperature of only around 950 ° C. As a result, the required working temperature is reduced considerably, which is what makes fused-salt electrolysis possible in the first place.
The working temperatures are maintained because, due to the electrical resistance of the melt, it also works like a resistance heater and supplies the supply heat for melting. Heat is constantly lost during the process:
- through the cooling endothermic reaction of the reduction ( enthalpy of reaction ),
- by sucking off the hot liquid aluminum (draining according to the suction lifter principle ),
- because the resulting reaction gases withdraw as hot exhaust gas.
The reduction of aluminum oxide takes place in the melt electrolysis (also abbreviated to melt electrolysis ). The electrolysis cell consists of a steel tub that is lined with carbon material ( graphite / anthracite ). The liquid electrolyte (cryolite with an excess of AlF 3 ) is located in this tank . The anodes ( graphite blocks burned from petroleum coke ), which are connected to the positive pole of a voltage source , dip into the electrolyte from above . The cathode well, on the other hand, is connected to the negative pole. In the melt, the aluminum oxide is present in its ions dissociated.
With a voltage of 4 to 5 volts and a current strength of up to 330,000 amperes (a current density of 8,000 amperes / square meter), the aluminum oxide (Al 2 O 3 ) is broken down with the help of the direct current source. The high amperage is necessary to produce significant amounts of aluminum on a large scale, because according to Faraday's law , the weight of an electrolytically formed substance is proportional to the electrical charge that has flowed and, last but not least, the heating according to Ohm's law requires such high currents.
The positively charged aluminum ions Al 3+ in the melt migrate to the cathode (negative pole). There they take up electrons and are reduced to aluminum atoms.
The negative oxygen ions O 2− migrate to the anode (positive pole). There they release their excess electrons , where they react with the carbon of the graphite anode to form carbon monoxide and carbon dioxide , which then escape as gases.
The resulting liquid aluminum has a greater density than the molten aluminum oxide-cryolite mixture and therefore collects on the bottom of the cathode pan. From there it is drawn off with a suction pipe. The pure aluminum produced in this way still contains around 0.1 to 1 percent impurities. These are essentially iron , silicon and titanium .
During this process, undesirable side reactions can occur, some of which represent major environmental problems. For example, significant amounts of hydrogen fluoride , carbon monoxide and fluoroalkanes, especially tetrafluoromethane, which increase the greenhouse effect , are produced .
Individual evidence
- ↑ a b c M. Binnewies et alii: Allgemeine und Anorganische Chemie. 2nd Edition. Spectrum, 2010, ISBN 3-8274-2533-6 . P. 404f.
- ↑ Energy efficiency in aluminum extraction (PDF; 1.8 MB) .
- ↑ Volkmar M. Schmidt: Elektrochemische Verfahrenstechnik , ISBN 978-3-527-62362-4
- ↑ Scriptum Elektrochemie (PDF; 1.3 MB) of the University of Siegen, page 184.
- ^ A b Charles E. Mortimer, Ulrich Müller : Chemistry . 9th edition. Thieme, 2007, ISBN 978-3-13-484309-5 .
- ↑ European Aluminum Association: Short report (English) .