Hexamethylcyclotrisiloxane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
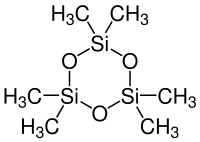
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexamethylcyclotrisiloxane | |||||||||||||||
| other names |
D 3 |
|||||||||||||||
| Molecular formula | C 6 H 18 O 3 Si 3 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 222.47 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.02 g cm −3 |
|||||||||||||||
| Melting point |
50-64 ° C |
|||||||||||||||
| boiling point |
134-135 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hexamethylcyclotrisiloxane ( D 3 ) is a chemical compound from the group of siloxanes . It is the simplest representative of the cyclic dimethylsiloxanes.
Extraction and presentation
From dimethyldichlorosilane produced by the Müller-Rochow synthesis , a mixture of linear and cyclic dimethylsiloxanes is obtained by hydrolysis , where D 4 is contained in the highest concentration. D 3 can also be distilled off from the mixture. If DMSO is used as the oxygen source instead of water, primarily cyclic dimethylsiloxanes are formed and D 3 predominates over D 4 . Hexamethylcyclotrisiloxane can also be obtained by heating higher molecular weight dimethylpolysiloxane to 350 ° C.
properties
Hexamethylcyclotrisiloxane is a moisture-sensitive, flammable, crystalline, white, odorless solid that decomposes in water. In contrast to larger cyclic dimethylsiloxanes, D 3 has a relevant ring strain , so that it reacts faster than other dimethylsiloxanes in both acidic and basic catalysis.
use
Hexamethylcyclotrisiloxane is used for the production of graft polymers and block polymers and is also used in biochemistry for proteome research and also functions as an intermediate in organic reactions. It can also be used as a starting material for plasma polymerizations . It is also used in cosmetics.
Occurrence
D 3 can be detected both in indoor air and in the environment (outside air and sewage sludge ).
Individual evidence
- ↑ a b c d e f g h i Entry on hexamethylcyclotrisiloxane in the GESTIS substance database of the IFA , accessed on November 18, 2016(JavaScript required) .
- ^ Philip L. Fuchs: Handbook of Reagents for Organic Synthesis, Reagents for Silicon-Mediated Organic Synthesis . John Wiley & Sons, 2013, ISBN 978-1-118-63613-8 , pp. 310 ( limited preview in Google Book search).
- ↑ a b Michael A. Brook: Silicon in Organic, Organometallic and Polymer Chemistry. Wiley: New York, 2000, ISBN 0-471-19658-4 , pp. 258-268.
- ↑ Google Patents: Patent EP0126792B1 - Process for the production of hexamethylcyclotrisiloxane and a use of the cyclotrisiloxane produced in this way , accessed on November 18, 2016.
- ↑ Data sheet Hexamethylcyclotrisiloxane, 97% from AlfaAesar, accessed on November 18, 2016 ( PDF )(JavaScript required) .
- ↑ Hans-Georg Elias: Macromolecules, chemical structure and syntheses . Sixth, completely revised edition. John Wiley & Sons, 2009, ISBN 3-527-62648-4 , pp. 364 ( limited preview in Google Book search).
- ↑ Google Patents: Patent DE10326899A1 - Cosmetic preparations with stabilized preservatives - Google Patents , accessed on November 18, 2016.
- ↑ Google Patents: Patent EP0118625A2 - Use of hexamethylcyclotrisiloxane as a fragrance carrier material - Google Patents , accessed on November 18, 2016.
- ↑ Mira Portmann: SILOXANE in the environment and in sewage gas , report October 2009, Office for Waste, Water, Energy and Air of the Canton of Zurich.
- ↑ Amelie Kierkegaard, Michael S. McLachlan: Determination of linear and cyclic volatile methylsiloxanes in air at a regional background site in Sweden . In: Atmospheric Environment . 80, 2013, pp. 322-329. doi : 10.1016 / j.atmosenv.2013.08.001 .
