Holmium (III) chloride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Ho 3+ __ Cl - | ||||||||||
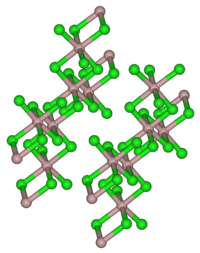
| Space group |
C 2 / m (No. 12) |
|||||||||
| General | ||||||||||
| Surname | Holmium (III) chloride | |||||||||
| other names |
Holmium trichloride |
|||||||||
| Ratio formula | HoCl 3 | |||||||||
| Brief description |
light yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 271.29 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
3.7 g cm −3 |
|||||||||
| Melting point |
|
|||||||||
| boiling point |
1470 ° C |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Holmium (III) chloride is a chemical compound from the group of chlorides .
Extraction and presentation
Holmium (III) chloride can be obtained by reacting holmium (III) oxide and ammonium chloride at 200–250 ° C.
The hexahydrate is formed by the reaction of holmium with hydrochloric acid . This can be converted to the anhydrate form by reaction with thionyl chloride .
Holmium (III) chloride can also be synthesized directly from the elements holmium and chlorine .
properties
Holmium (III) chloride and its hexahydrate are pale yellow solids in daylight that are soluble in water. The hexahydrate begins to give off water of crystallization at 64 ° C. Holmium (III) chloride has a monoclinic crystal structure similar to that of aluminum (III) chloride .
use
Holmium (III) chloride is used to make pure holmium.
Individual evidence
- ↑ a b c d Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists . 2007, ISBN 978-3-540-60035-0 , pp. 488 ( limited preview in Google Book search).
- ↑ a b c d e f data sheet Holmium (III) chloride, anhydrous, powder, 99.9% trace metals basis from Sigma-Aldrich , accessed on April 30, 2012 ( PDF ).
- ^ Americanelements: Holmium Chloride
- ↑ Data sheet Holmium (III) chloride hexahydrate, 99.9% trace metals basis from Sigma-Aldrich , accessed on April 30, 2012 ( PDF ).
- ↑ a b c Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY.
- ↑ Web elements: Holmium
- ^ John Emsley: Nature's building blocks: an AZ guide to the elements . Oxford University Press, 2003, ISBN 978-0-19-850340-8 , pp. 181 ( limited preview in Google Book search).



