IAEDANS
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
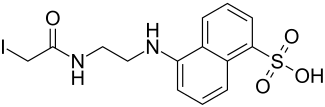
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | IAEDANS | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 15 IN 2 O 4 S | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 434.25 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
> 300 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
IAEDANS is an organic fluorescent dye , in the molecular biology frequently for marking of proteins is used because it easily with thiol groups of the amino acid cysteine reacts and in the neutral pH range is readily soluble in water. Since the fluorescence is highly dependent on the solution and the environment, it is very suitable for bonding measurements by changing the polarization of the emitted radiation or by FRET . The dye is excited by ultraviolet radiation at a wavelength of 336 nm and emits in the range around 490 nm. The absorption at 336 nm takes place with an extinction coefficient of 5700.
IAEDANS was first synthesized in the early 1970s by biochemist Gregorio Weber , and in 1973 the optical properties and synthesis were described.
Individual evidence
- ↑ IAEDANS data sheet at Acros, accessed on February 26, 2010.
- ↑ Data sheet N- (Iodoacetaminoethyl) -1-naphthylamine-5-sulfonic acid from Sigma-Aldrich , accessed on October 17, 2016 ( PDF ).
- ↑ Earl N. Hudson, Gregorio Weber: Synthesis and characterization of two fluorescent sulfhydryl reagents. In: Biochemistry. Volume 12, No. 21, 1973, pp. 4154-4161, doi : 10.1021 / bi00745a019 .