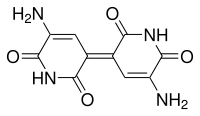
Indigoidine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Indigoidine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 10 H 8 N 4 O 4 | |||||||||
| Brief description |
blue powder |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 248.19 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| solubility |
almost insoluble in water, soluble in 6N HCl |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Indigoidin is an organic compound belonging to the aza quinones group . It is a blue pigment that some types of bacteria form and excrete into the surrounding medium.
history
Otto Voges researched and described the bacterial species Bacillus indigoferus in Kiel as early as 1893 , renamed after him Vogesella indigofera , which continuously discolored the surrounding medium (water) from a light bluish (24 hours) to royal blue (48 hours).
In 1964 and 1965, Nobel Prize winner Richard Kuhn and his colleagues published several articles in the specialist press on the occurrence, structure and synthesis of indigoidin.
In 1979 Carl-Gerd Dieris and H.-D. Sharp the synthetic production of indigoidine.
Occurrence
The indigoid is found in the following types of bacteria:
- Arthrobacter atrocyaneus
- Arthrobacter crystallopoietes
- Arthrobacter polychromogones
- Corynebacterium insidiosum
- Erwinia chrysanthemi was renamed Dickea dadantii
- Vogesella indigofera formerly Bacillus indigoferus and Pseudomonas indigofera
Extraction and manufacture
Indigoidin can be produced synthetically from citric acid (C 6 H 5 NO 4 ), which is easily accessible from citric acid and ammonia .
biosynthesis
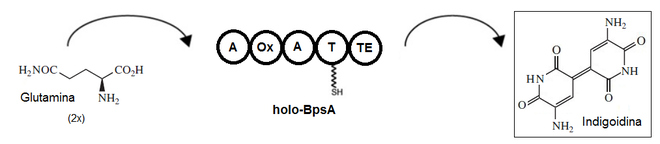
The biosynthesis of indigoidin starts from glutamine , which is enzymatically oxidized , cyclized to form a heterocycle and dimerized .
The details of the segments A, Ox, A, T and Te relate to defined domains within the enzyme structure of the bpsA gene product BPSA.
properties
Physical Properties
Indigoidin is a blue amorphous powder with a molar mass of 248.19 g · mol −1 . It is insoluble in water and most other solvents, but it dissolves in 6N hydrochloric acid with a royal blue color and in hot sulfuric acid with an orange-brown color.
Chemical properties
The color of indigoid is attributed to an indigoid chromophore . The NMR spectroscopic investigations on derivatives confirmed the specified symmetrical structure. Because of the nitrogen atoms in the quinoid rings, indigoidin is assigned to the compound class of aza quinones .
Derivatives
One derivative is the violet dye N 5 , N 5 ′ -Didodecylindigoidin , (C 34 H 56 N 4 O 4 ), which was isolated from the psychrophilic bacterium Shewanella violacea DSS12.
See also
- Indigo - C 16 H 10 N 2 O 2
- Indigotine I - C 16 H 8 N 2 Na 2 O 8 S 2
- Royal blue - inorganic pigments
literature
- Carl-Gerd Dieris: On the question of the luminescence of thermooxidatively damaged polycarpolactam. A new synthesis of the bacterial dye indigoidin and its tetra-N-alkyl derivatives . 1980.
- Hans Günter Schlegel : General microbiology . Thieme Publishing Group , Stuttgart 1992, ISBN 978-3-13-444607-4 .
- Sylvie Reverchon, Carine Rouanet, Dominique Expert, William Nasser: Characterization of Indigoidine Biosynthetic Genes in Erwinia chrysanthemi and Role of This Blue Pigment in Pathogenicity . In: Journal of Bacteriology . tape 184 , no. 3 , January 2, 2002, p. 654-665 , doi : 10.1128 / JB.184.3.654-665.2002 , PMID 11790734 .
- Christin Schönfeld: Characterization and biochemical analysis of the indigoidin synthease BpsA from S. lavendulae ATCC 11924 . Master thesis , Philipps University Marburg 2012.
- M. Müller, S. Ausländer, D. Ausländer, C. Kemmer, M. Fussenegger: A novel reporter system for bacterial and mammalian cells based on the non-ribosomal peptide indigoidine . Metabolic Engineering 14/2012, pp. 325-335 ( doi: 10.1016 / j.ymben.2012.04.002 ).
- H. Kobayashi, Y. Nogi, K. Hirokoshi: New violet 3,3'-bipyridyl pigment purified from deep-sea microorganism Shewanella violacea DSS12 . In: Extremophiles No. 11 (2) / 2012, pp. 245–250. PMID 17102923 .
Individual evidence
- ^ RA Abramovitch: The Chemistry of Heterocyclic Compounds. Pyridine and Its Derivatives . John Wiley & Sons , New York 1974, ISBN 0-471-37915-8 , p. 860.
- ↑ a b entry on indigoidine. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Richard Kuhn, Helmut Bauer, Hans-Joachim Knackmuss, Daisy A. Kuhn, Mortimer P. Starr: The structure of the blue pigments of Corynebacterium insidiosum, Arthrobacter atrocyaneus, Pseudomonas indigofera and Arthrobacter crystallopoietes . In: Natural Sciences . tape 51 , no. 17 , January 1, 1964, p. 409 , doi : 10.1007 / BF00609040 .
- ^ Richard Kuhn, Helmut Bauer, Hans-Joachim Knackmuss: Structure and syntheses of the bacterial dye indigoidin . In: Chemical Reports . tape 98 , no. 7 , 1965, pp. 2139-2153 , doi : 10.1002 / cber.19650980714 .
- ↑ Richard Kuhn, Mortimer P. Starr, Daisy A. Kuhn, Helmut Bauer, Hans-Joachim Knackmuss: Indigoidine and other bacterial pigments related to 3,3'-bipyridyl . In: Archives for Microbiology . tape 51 , no. 1 , March 1, 1965, p. 71-84 , doi : 10.1007 / BF00406851 .
- ↑ C.-G. Dieris, H.-D. Sharp: A new synthesis of the bacterial dye indigoidin and its tetra-N-alkyl derivatives . In: Synthesis . tape 1979 , no. 12 , 1979, pp. 948-950 , doi : 10.1055 / s-1979-28883 .
- ↑ M. Müller, S. Ausländer, D. Ausländer, C. Kemmer, M. Fussenegger: A novel reporter system for bacterial and mammalian cells based on the non-ribosomal peptide indigoidine . In: Metabolic Engineering . No. 14 , 2012, p. 325-335 , doi : 10.1016 / j.ymben.2012.04.002 .
- ↑ Hitoshi Takahashi, Takanori Kumagai, Kyoko Kitani, Miwako Mori, Yasuyuki Matoba and Masanori Sugiyama: Cloning and Characterization of a Streptomyces Single Module Type Non-ribosomal Peptide Synthetase Catalyzing a Blue Pigment Synthesis , J. Biol. Chem. 282 (12), Pp. 9073-9081 (2007).
- ↑ H. Kobayashi, Y. Nogi, K. Hirokoshi: New violet 3,3'-bipyridyl pigment purified from deep-sea microorganism Shewanella violacea DSS12 . In: Extremophiles . No. 11 (2) , 2007, pp. 245-250 .