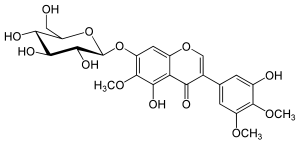
Iridine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Iridine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 24 H 26 O 13 | ||||||||||||
| Brief description |
colorless needles |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 522.46 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
208 ° C |
||||||||||||
| solubility |
soluble in water and alcohol |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Iridin is a glucoside of irigenin.
Occurrence
Iridin and its aglycon irigenin belong to the isoflavones and occur together in the roots of iris species ( Iris florentina , Iris germanica , Iris pallida ) and in Belamcanda chinensis .
Chemistry and properties
Iridine forms fine, white needles that melt at 208 ° C and dissolve well in water and ethanol. It is chemically a glycoside with the isoflavone derivative irigenin as an aglycon, to which glucose is O -glycosidically bound. The name iridin must not be confused with the natural product group of iridoids , as their biosynthesis is different.
Other names
A soft resin from the alcoholic extract of Iris versicolor and a protamine from the sperm of rainbow trout are also referred to as iridin .
Individual evidence
- ↑ a b c d Entry on Irish woman. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ a b E. A. Schmidt: Detailed textbook on pharmaceutic chemistry. 2nd edition, Vieweg, 1901.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Atta-ur-Rahman, S. Nasim, I. Baig, I. Ara Jahan, B. Sener, I. Orhan, MI Choudhary: Isoflavonoid glycosides from the rhizomes of Iris germanica. Chem Pharm Bull , 50 , 2002 , 1100-1102. doi : 10.1248 / cpb.50.1100
- ↑ T. Akashi, M. Ishizaki, T. Aoki, S. Ayabe: Isoflavonoid production by adventitious-root cultures of Iris germanica (Iridaceae). Plant Biotechnology 22 , 2005 , 207-215. doi : 10.5511 / plantbiotechnology.22.207

