Isopropyl nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
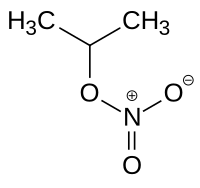
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isopropyl nitrate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 7 NO 3 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 105.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.04 g cm −3 (20 ° C) |
|||||||||||||||
| boiling point |
101 ° C |
|||||||||||||||
| Vapor pressure |
36 h Pa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| Refractive index |
1.391 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−229.7 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Isopropyl nitrate , also called IPN, is the nitric acid ester of isopropanol , a colorless to yellowish, oily liquid.
properties
In its pure form and in the liquid state, isopropyl nitrate can hardly be detonated, but the vapor / air mixtures are highly explosive and react much more violently than normal fuel / air mixtures. At room temperature it is an oily liquid with a pungent sharp, but also sweet, fruity odor. The fumes are similarly toxic as most other short chain alkyl nitrates, such as methyl nitrate and ethyl nitrate . After prolonged exposure, they usually cause headache and drowsiness and, in the event of chronic exposure, damage the internal organs, especially the lungs and liver, and the nervous system.
presentation
Isopropyl nitrate is produced by esterifying isopropanol with nitric acid or, better, nitrating acid. An autocatalyzed side reaction is the oxidation of isopropanol to acetone with the formation of nitrogen oxides, which is much more exothermic than the nitration reaction and can proceed in an uncontrolled manner if there is insufficient temperature control and high nitric acid concentrations.
use
IPN is used primarily as a rocket and turbine fuel and as an additive for diesel fuel . The Russian rocket launchers RPO-A Schmel and TOS-1 use IPN as a component of the thermobaric warheads.
Individual evidence
- ↑ a b c d e f g Entry on isopropyl nitrate in the GESTIS substance database of the IFA , accessed on October 8, 2012(JavaScript required) .
- ↑ Isopropyl nitrate data sheet from Sigma-Aldrich , accessed on May 15, 2017 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
